When cannabis is heated at a low temperature for a long period of time, cannabinoids THCA and CBDA are activated and transformed into THC and CBD. This process is called Decarboxylation and is used to prepare cannabis for cooking and create higher potency products for oral consumption.
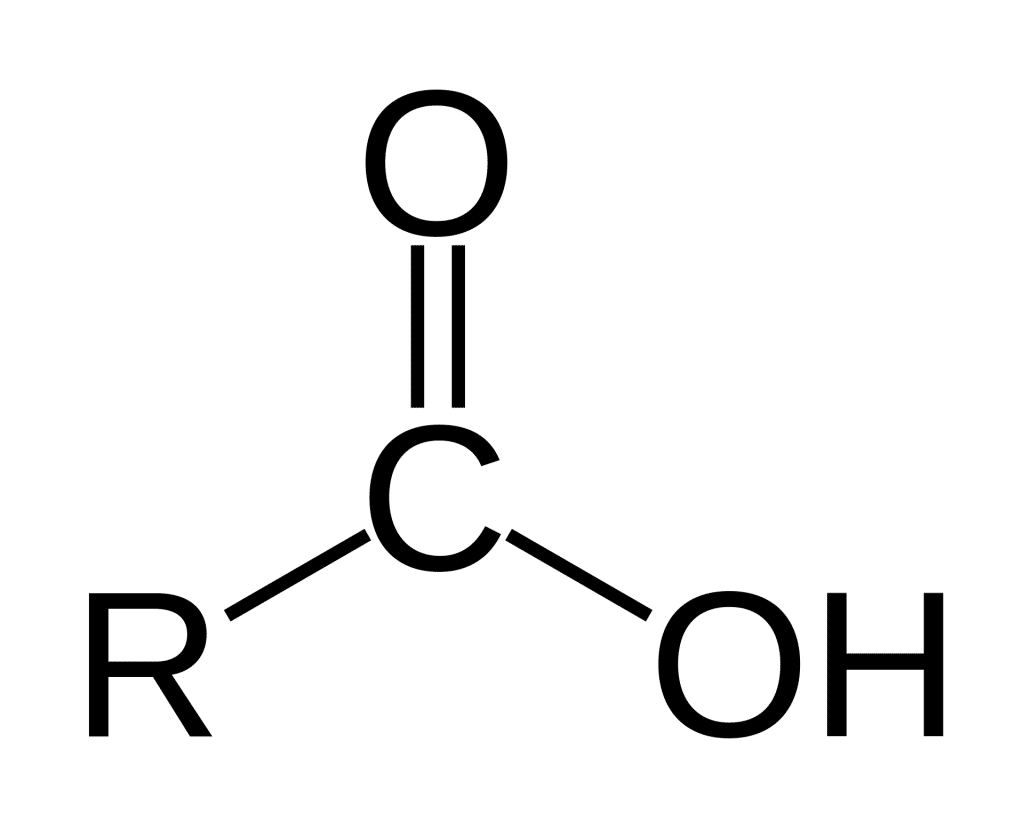
Scientists have an intense and burning need to name stuff. Not just stuff, but the stuff that makes up that stuff. Chemists are no exception to that. In many ways, they are the worst offenders among us (IUPAC Nomenclature having induced anxiety in an unknown number of students so far). Over the past 250 years, chemists have codified a list of common molecular motifs, kind of like naming Lego pieces which you use all the time. They are called functional groups, and they’re identified by name, chemical abbreviation, and skeletal structure. These include alcohols (R—OH), aldehydes (RCHO), ethers (R—O—R’), and—most relevantly—carboxylic acids (RCO2H).
Carboxylic acids are so called because they are weak acids that decrease the pH of a solution by shedding their hydrogen atom (their proton), and because they are composed of carbon and oxygen atoms (carb and oxy).
Carboxylic acids are important to the understanding of cannabis science because cannabinoids are almost exclusively biosynthesized in what is known as their ‘acidic form.’ [1] That’s what the ‘A’ stands for in THCA, CBDA, CBGA — ‘Acid’. But really, the ‘A’ stands for ‘carboxylic acid’ because that’s what the acidic forms of all the cannabinoids contain.
Decarboxylation is the act of chemically modifying a molecule by removing its carboxylic acid without affecting any other facet of its chemical composition. Decarboxylation is a required step to ‘activate’ the physiological and psychedelic effects of most cannabinoids.
In the cannabis industry, you’ll find decarboxylation is almost exclusively done by the application of heat. This is because the core of a carboxylic acid is a molecule of carbon dioxide—CO2—which naturally exists as a gas and has relatively little problem going back to doing so.
Cannabis products whose consumption does not involve heat (such as by edible ingestion), or which operate at lower temperatures than required for cannabinoid decarboxylation (such as vape pens), must therefore have their cannabinoids previously activated in the manufacturing process.
References
- Flores-Sanchez and Verpoorte. “Secondary Metabolism in Cannabis.” Phytochemistry Reviews, vol, 7, no. 3, 2008, pp. 615-39. [Times cited = 83; Journal impact factor = 1.064].