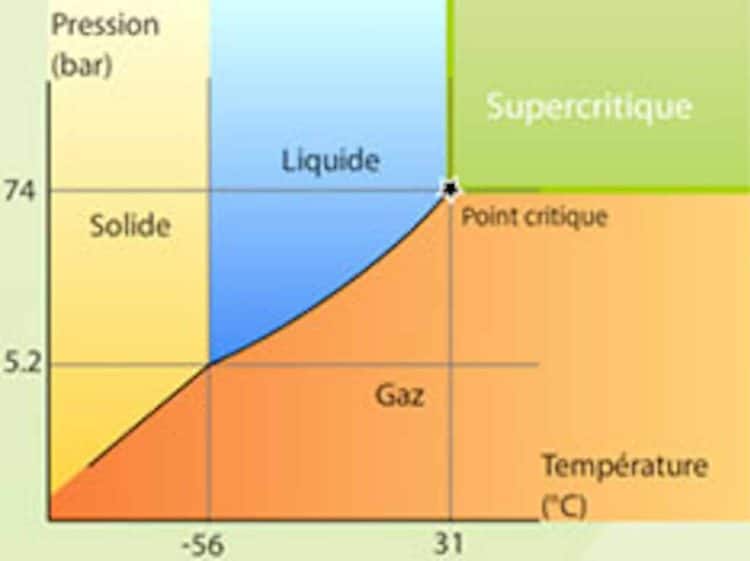
Supercritical fluid extraction (SFE) is a method of separating different constituents of a material or substance through the use of a particular solvent in a supercritical state. “Supercritical”refers to a molecular condition that occurs when a compound is exposed to temperatures and pressures above its critical point [1]. A substance in a supercritical state will display properties that are both gas-like and fluid-like, resulting in a viscosity comparable to gas and a density comparable to liquid.
SFE is a popular cannabis extraction technique due to its ability to extract desired compounds from the plant while leaving behind undesirable components. The extracted oil isn’t contaminated with residual hydrocarbon solvents, either. When we talk about SFE in cannabis extraction, we’re talking carbon dioxide (CO2). The critical point of CO2 is 31 °C with a pressure of 73 bar [2]. Supercritical CO2 is unique as a solvent because it possesses tunability to isolate specific elements of plant material due to the relationship between the density of the supercritical CO2, and the varying solubilities of the phytochemicals. By choreographing the temperature and pressure conditions, the density of the supercritical CO2 can be tailored to target specific molecules. This is really the take-home message regarding CO
The below table illustrates the properties of water, ethanol, methanol, and carbon dioxide at their critical states [3]. As can be seen, the low critical temperature of CO2 presents an attractive option, since it means labile, volatile molecules can be isolated without degradation. Thus, delicate terpenes can be more easily retained.
| Property | Water | Methanol | Ethanol | CO2 |
|---|---|---|---|---|
| Critical temperature, Tc/K | 647.0 | 512.6 | 514.1 | 304.2 |
| Critical temperature, tc/°C | 374.0 | 239.5 | 241.0 | 31.0 |
| Critical pressure, Pc/MPa | 22.1 | 8.1 | 6.1 | 7.4 |
| Critical pressure, Pc/atm | 217.8 | 79.9 | 60.6 | 72.8 |
| Critical density, ρc/kg m-3 | 322 | 272 | 276 | 468 |
References
- Petrucci, R. et al. The Critical Point. General Chemistry: Principles & Modern Applications, ninth Edition. New Jersey: Pearson Education, Inc., 2007. 478-479.
- Marcus, Y. “Some Advances in Supercritical Fluid Extraction for Fuels”, Bio-Materials and Purification Processes, 2019, Volume 7: 156. [Journal Impact Factor = 2.973]
- Chen, E. et al. Continuous production of biodiesel via supercritical methanol transesterification in a tubular reactor. Part 1: Thermophysical properties and transitive properties of supercritical methanol. Energy Fuels, 2009, Volume 23: 526–532. [Journal Impact Factor = 3.091 Times Cited = 23]