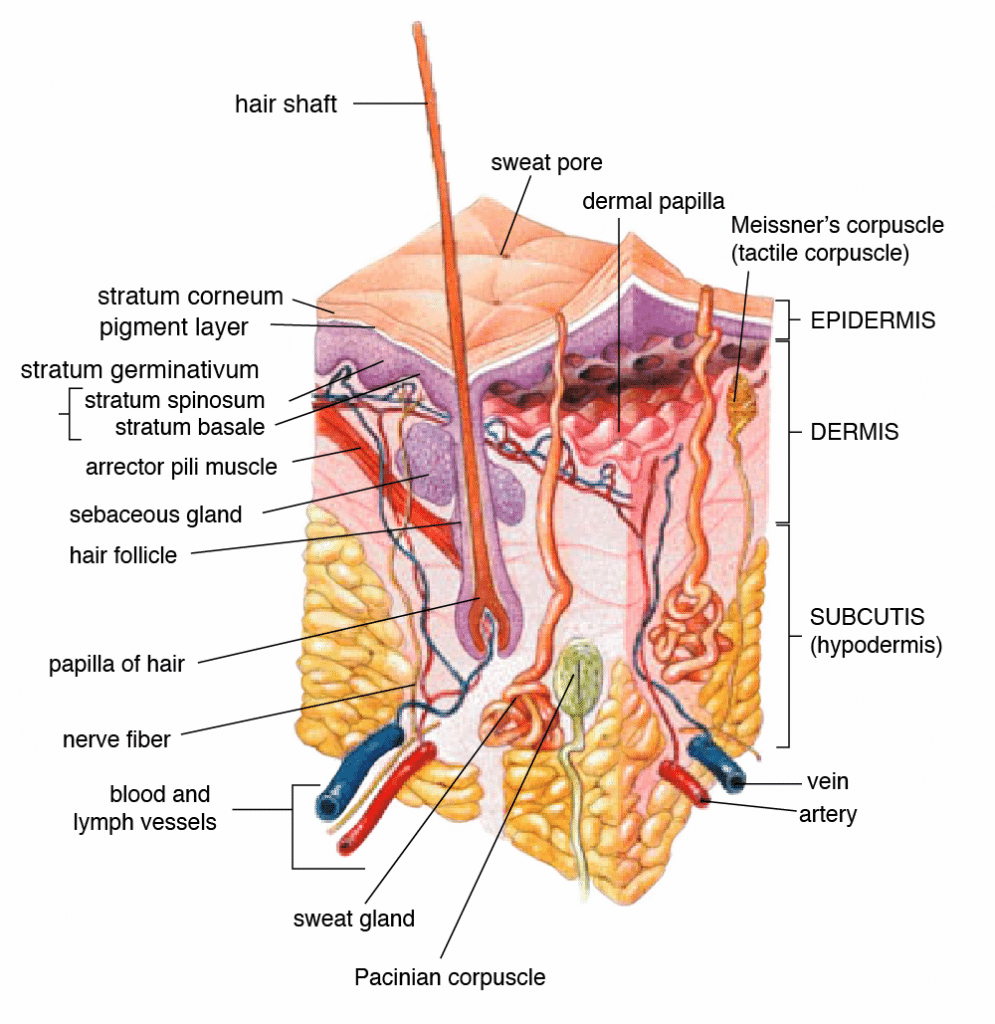
Human skin serves three major purposes: homeostasis, sensation, and protection. The multiple complex structures of the skin make it the largest organ of the human body at roughly 15% of adult body weight. [1,2] Topical and transdermal applications that deliver active ingredients via the skin have been utilized since ancient times; today, they account for 15-20% of all repeat prescriptions. [3] However, there are significant differences between topical and transdermal products.
Topical applications affect the epidermis (upper most skin layer) and to a lesser extent the dermis (second main skin layer). Topical products act locally and do not fully penetrate the dermis into subcutaneous tissue. The active ingredients in a topical application effectively act on the skin itself. They do not enter blood vessels or proliferate to systemic circulation. Examples in the pharmaceutical realm include ointments designed to treat acne and psoriasis. [4,5] A topical cannabis product may be purposed to reduce localized joint pain or skin issues.
The skin serves as a barrier against external pathogens and environmental threats. The stratum corneum of the epidermis blocks molecular transport of most topical agents as a matter of mechanical protection. [1,6] The stratum corneum also has an extracellular lipid matrix that safeguards against chemical invasion. [7] In the case of a topical, ingredients permeate this barrier poorly through hair follicles and sweat glands. [4]
Transdermal applications increase skin permeability to the point that active ingredients can fully penetrate the epidermis and dermis to enter systemic circulation. Transdermal patches and products may be used to circumvent the degradation of medicinal compounds in the gastrointestinal tract and liver via first-pass metabolism. They are a convenient and potentially superior alternative to oral administration of pharmaceuticals. [4,6] Transdermal cannabis could hypothetically replace ingestion, vaporization, or combustion. Location of transdermal application is relatively insignificant. One example is the nicotine transdermal patch to help tobacco smokers quit.
The principle challenge associated with transdermal delivery is penetrating the stratum corneum. It is effective against hydrophilic (water-friendly) compounds but less so against lipophilic compounds (lipid-friendly), so manufacturers must ensure lipophilicity. [8,9] Drug Delivery reports that passive applications require active ingredients with molecular weights of 500 daltons or less. [9] Nanoscale liposomes have demonstrated viability; these lipid-based vesicles permeate skin and deliver active ingredients as colloidal dispersions. [8] However, chemical enhancers may incite skin irritability. Other methods under investigation are ultrasound, microneedles, abrasion, and high-voltage pulses. [6,9]
Whether consumer or manufacturer, it is imperative to comprehend the difference between the terms topical and transdermal. While both refer to the skin, topical products target the skin and local areas of the body while transdermal products penetrate the skin for systemic effects.
References
- Kolarsick, Paul, et al. “Anatomy and Physiology of the Skin.” Journal of the Dermatology Nurses Association, vol. 3, no. 4, 2011, pp. 214–215., doi:10.1097/jdn.0b013e31822bdc94. Journal Impact Factor = 0.02. Cited by = 121 (GoogleScholar)
- Kanitakis, J. “Anatomy, Histology and Immunohistochemistry of Normal Human Skin.” European Journal of Dermatology, 12, no. 4, 2002, pp. 390-401. Journal Impact Factor = 1.944 Cited by = 41 (PubMed)
- Alany, Raid. “Topical and Transdermal Formulation and Drug Delivery.” Pharmaceutical Development and Technology, vol. 22, no. 4, 2017, pp. 457–457., doi:10.1080/10837450.2017.1310175. Journal Impact Factor = 1.945. Cited by = 1 (GoogleScholar)
- Ruela, André Luís Morais, et al. “Evaluation of Skin Absorption of Drugs from Topical and Transdermal Formulations.” Brazilian Journal of Pharmaceutical Sciences, vol. 52, no. 3, 2016, pp. 527–544., doi:10.1590/s1984-82502016000300018. Journal Impact Factor = 0.483 Cited by = 14 (ResearchGate)
- Ueda, et al. “Topical and Transdermal Drug Products.” Pharmacopeial Forum, vol. 35, 2009, pp. 750-764. 10.14227/DT170410P12. Journal Impact Factor = 0.21 Cited by = 61 (ResearchGate)
- Prausnitz, Mark R., et al. “Current Status and Future Potential of Transdermal Drug Delivery.” Nature Reviews Drug Discovery, vol. 3, no. 2, 2004, pp. 115–124., doi:10.1038/nrd1304. Journal Impact Factor = 57.000 Cited by = 121 (PubMed)
- Murphrey MB, Zito PM. “Histology, Stratum Corneum.” StatPearls [Internet], Treasure Island, FL: StatPearls Publishing, 2019. https://www.ncbi.nlm.nih.gov/books/NBK513299/
- Hua, Susan. “Lipid-Based Nano-Delivery Systems for Skin Delivery of Drugs and Bioactives.” Frontiers in Pharmacology, vol. 6, 2015, doi:10.3389/fphar.2015.00219. Journal Impact Factor = 4.400 Cited by = 5 (PubMed)
- Brown, Marc B., et al. “Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects.” Drug Delivery, vol. 13, no. 3, 2006, pp. 175–187., doi:10.1080/10717540500455975. Journal Impact Factor = 4.843 Cited by = 38 (PubMed)