Modern cannabis concentrate manufacturers are increasingly turning toward carbon dioxide (CO2) extractions. It’s a non-toxic, environmentally-friendly way to create potent concentrates saturated with cannabinoids and terpenes.
Supercritical CO2 extractions are the most common, but they’re not the sole method that professionals use. Subcritical CO2 extractions are also performed. The differing methods accomplish different goals.
How Does it Work?
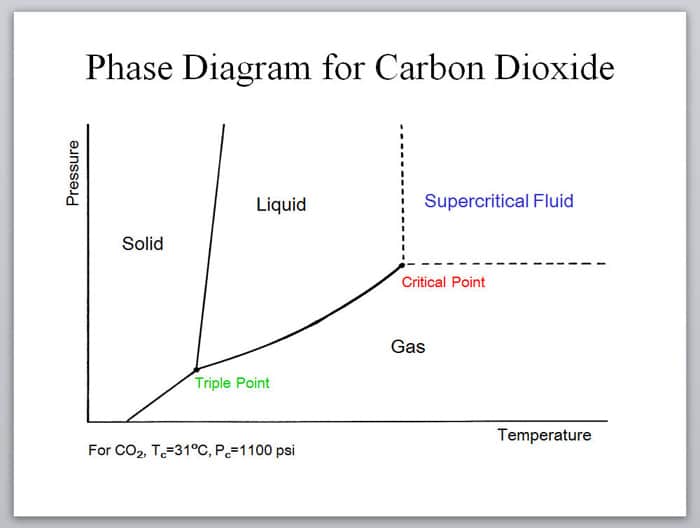
At certain pressure and temperature ranges, CO2 behaves like a solvent and can be used to pull specific compounds from plant material.
A “supercritical” solvent exhibits the features of both a liquid and a gas. It can slide into porous materials as well as dissolve them.
“The solvent power of a supercritical fluid is approximately proportional to its density,” Susan Bell of SRI Consulting notes in a report published by Chemical Engineering. “Thus, solvent power can be modified by varying the temperature and pressure. Because their properties are a strong function of temperature and pressure, supercritical fluids are considered tunable solvents.”
That’s why CO2extractions are useful in the cannabis industry. The “tunability” of the solvent means that specific compounds can be targeted.
Entering into the supercritical state depends on both temperature and pressure. Any combination of these that puts the system over the “critical point” will cause CO2 to become supercritical. In most cases, CO2 must reach pressures above 1,000 psi just to enter the supercritical fluid state. Many CO2 extraction methods call for pressures significantly higher (1,600-4,000 PSI).
Subcritical vs Supercritical CO2
Subcritical CO2 extractions require less pressure and a lower temperature than supercritical ones. The process is longer and less efficient, and the typical yield is smaller.
In a subcritical extractions, the pressure is maintained while the temperature is taken below the supercritical threshold, causing it to become a (non-supercritical) liquid. It still maintains some of hydrophobic extraction properties, but the lower temperatures protect fragile constituents from denaturing.
Supercritical extractions are too harsh for some terpenes.1 Monoterpenes are typically destroyed while sesquiterpenes and terpene alcohols are concentrated. Alexander Beadle for Analytical Cannabis writes: “Many monoterpenes have relatively low vapor pressures and it is thought that the moderate heat involved… may have caused a loss or a transformation of these compounds.” However, they produce a greater yield and take less time.
References:
- Sexton, M, et al, “Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate”, Planta Med, 2018, Volume 84.