How Does it Work?
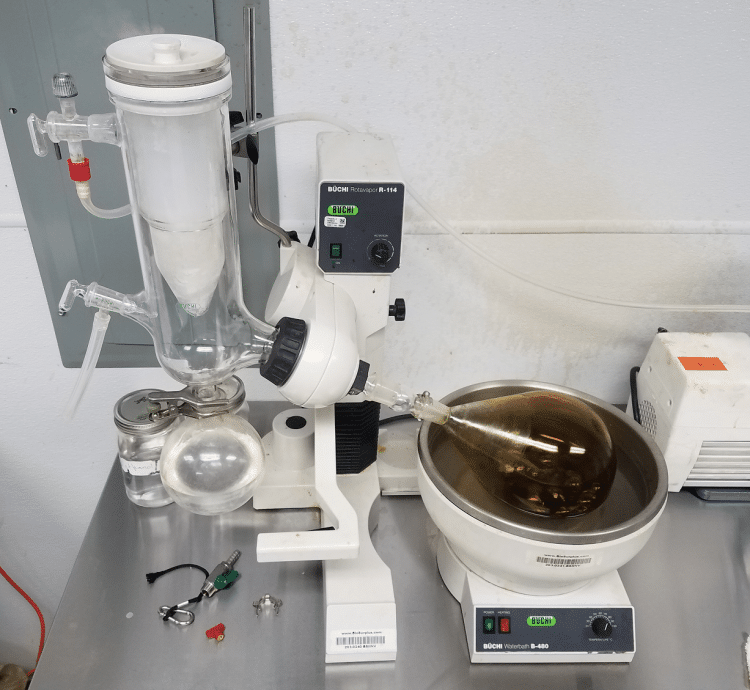
A rotary evaporator, or “rotovap” in lab jargon, is an instrument used in a chemistry lab to remove a volatile solvent from a liquid mixture. In a cannabis setting, the most common use for it is to remove ethanol from a winterized extract.
The rotary evaporator functions on the general assumption that the boiling point of the solvent is much lower than that of the liquid surrounding it. It also assumes the compounds in the liquid are thermally sensitive; otherwise you could just boil the solvent out.
A rotovap works by creating a vacuum in a rotating glass flask. What does that accomplish? Let’s go back to high school physics and chemistry.
All the matter in the universe exists in three (technically four) states: solid, liquid, gas, and plasma. Which state of matter a molecule is in co-depends on A) the physical conditions of the system it is in, and B) its intrinsic chemical properties. Of the physical variables, the most important to us are changes in temperature and pressure. Most industrial extractions procedures take place inside solid containers whose walls do not expand or contract (we hope); therefore, changes in volume are considered negligible.
Pressure and temperature are related to each other in a linear fashion; doubling the temperature (on the scale of absolute zero, or Kelvin scale) will results in a doubling of the pressure (so long as the volume is held constant). In effect, that means that reducing the pressure by turning on a vacuum also lowers the boiling point required for the volatile solvent to evaporate.
The evaporated solvent is captured by a “cold-finger”, a cooled surface which the gas molecules can encounter and condense upon. The most common way to make a cold-finger is to mix isopropyl alcohol and dry ice. Dry ice needs to be added continuously, but it’s not cold enough to freeze the alcohol and, unlike ice, it evaporates into gas when it is spent.
Simultaneous to all of the above, the flask is placed into a heated water bath and rotated continuously. This creates a thin film inside the flask that increases the surface area from which molecules of gas can evaporate. It also serves to conduct heat into the system directly, so the temperature remains constant even as molecules evaporate off.
As the vacuum is established and the newly-formed molecules of gas begin to condense on the cold-finger, the conditions of the system are such that evaporation proceeds simultaneously from every surface—even from inside the liquid itself. This leads to bubbling, which is the third reason why the flask is evaporated on a rotary motor.
Using a rotovap is both simple and effective, and is a tool you will see throughout your career either as a chemist or as a cannabis extraction tech.