Decarboxylation
Following last week’s article, here we present the second part of our “Methods for Extraction Oil Refinement” series, delving into some of the ways that cannabis extract manufacturers post-process their extracts.
verb: Decarb
As recently written about in our concurrently running series, “Extraction Terms, Demystified,” cannabinoids are almost always biosynthesized as acidic species with a carboxylic acid functional group—composed of two oxygen, one hydrogen, and one carbon atom—attached to the cannabinoid (RCO2H). Recent studies suggest that the acidic forms of cannabinoids produce novel physiological events that may have medicinal effects unique to those of their more well-known counterparts. [1-2] However, they are decidedly non-psychoactive. To “activate” their psychoactivity, the carboxylic acid of the cannabinoids must be removed. In other words: the cannabinoids need to be decarboxylated.
Every psychoactive cannabis product on the market has to be decarboxylated at some point before it enters the body because decarboxylation is required to unlock its psychoactive effects. However, not all cannabis products require decarboxylation prior to use: e.g., cannabis flower is never decarbed, always fresh. This is because the decarboxylation of cannabinoids is temperature dependent, and generally speaking, any method of cannabis consumption that operates in temperature ranges above 200°C (e.g., smoking, dabbing, etc.) will decarboxylate and vaporize the THC into gas nearly simultaneously. [3]
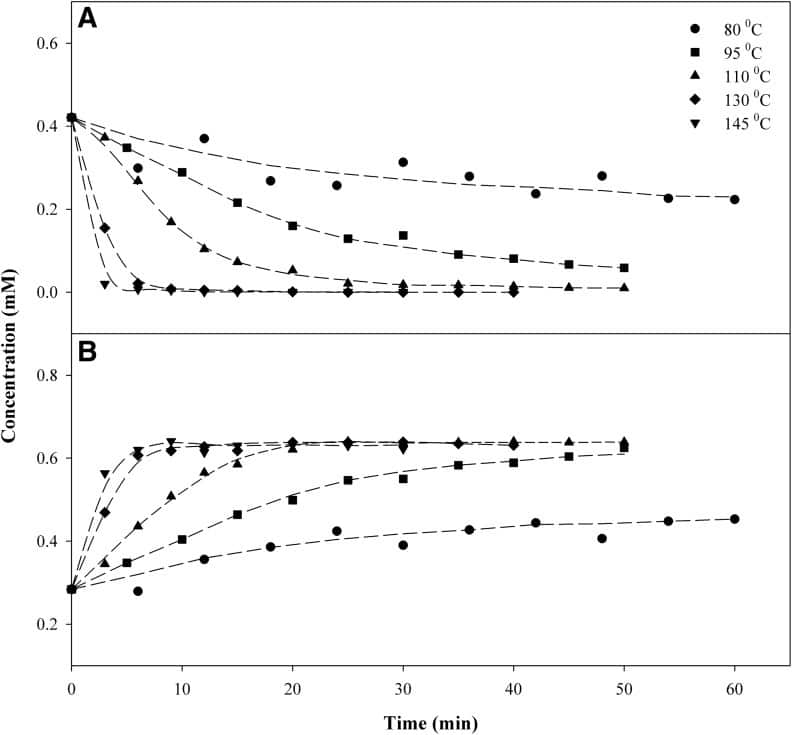
For the purposes of post-processing, at a given constant temperature, the amount of time acidic cannabinoid species (e.g., THCA) take to decarboxylate to neutral cannabinoid species (THC) will follow a predictable kinetic pattern, from which we can derive an empirical reaction rate. For example, looking at the figure below, we can deduce that at 110°C (230°F), complete decarboxylation of THCA to THC should occur within 30 minutes. [4]
Decarboxylations typically take place in a vacuum oven to minimize the oxidation of THC into CBN. [4] Even so, there are both minimum and maximum temperature limits to decarbing in an industrial setting. Temperatures lower than 85°C will produce impractically slow decarb times, while turning up the heat past 157°C will lead to THC vaporization and product loss. [3] There is an inverse relationship between how hot the decarb is done and the amount of final product you get to keep, because invariably, higher temperatures lead to higher loss.
While decarbing isn’t part of production for many cannabis products, there are many others for which it is a requirement. Vape pens heat up just past the point where THC vaporizes into gas, which is an insufficient temperature to decarboxylate the cannabinoids simultaneously. Many other products don’t use heat at all, e.g., dermal patches, oromucosal sprays, and certain edibles. These all require that decarboxylation occur as part of the post-processing step in production.
References
- Nadal, X. et al. “Tetrahydrocannabinolic Acid is a Potent PPARγ Agonist with Neuroprotective Activity.” Br J Pharmacol, vol. 174, no. 23, 2017, pp. 4263–4276. [Times cited = 16, Journal impact factor = 6.810]
- Palomares, B. et al. “Tetrahydrocannabinolic Acid a (THCA-A) Reduces Adiposity and Prevents Metabolic Disease Caused by Diet-Induced Obesity.” BioRxiv 622035 [Preprint]. April 29, 2019 [cited 2019 Aug 27]. Available from: https://doi.org/10.1101/622035
- Iffland, K. et al. “Decarboxylation of Tetrahydrocannabinolic Acid (THCA) to Active THC.” European Industrial Hemp Association, Oct. 2016. Accessed 27 Aug. 2019.
- Wang, M. et al. “Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry.” Cannabis Cannabinoid Res, vol. 1, no. 1, 2016, pp. 262–271. [Times cited = 27, Journal impact factor = N/A]
Image Credit: Max Pixel