The availability of delta-8-THC consumer products breathed new life into the hemp industry in 2020. Sales exploded throughout 2021 and the public’s enthusiasm for the hemp-derived psychoactive cannabinoid spurred the commercial development of related substances like HHC, THC-O, delta-10 and delta-9 from hemp. You can be sure that additional substances are being prepared from CBD as you read this article. Synthetic organic chemists are throwing everything but the kitchen sink at CBD in their search for their next big payday in the marketplace.
Sounds great, right? Building the industry with new and exciting products that meet the public’s demand is how any industry grows, but the news is not all favorable when it comes to delta-8 and other CBD conversion products. At the time of this writing, twelve states have banned the psychoactive substances obtained from converting CBD, with regulations limiting how they are sold in five other states. The reasons given for these bans aren’t so clear and vary from state to state. Some states ban them based on their psychoactive properties, while others claim these substances are unsafe, potentially laden with harmful contaminants. But what’s the truth? I consider myself somewhat knowledgeable about these matters, but reliable information is spotty and dependent on the source. For instance, a conflict of interest might exist with the publishing source, or the source of information is a consumer group favoring only cannabis derived delta-9 products. Sometimes the source is simply an anti-cannabis politician seizing on an opportunity to deal a black eye to our industry. I needed to speak to experts at a hemp analysis lab to review the available evidence and learn its significance. I needed to find laboratory personnel who had done their own research and reached their own conclusions, based on their examination of the scientific data.
I climbed into my car, buckled up and pointed it South for the three-hour drive to KCA Laboratories, located in the town of Nicholasville, Kentucky. There, I would meet with Dr. Richard Sams, Scientific Director, and Ryan Bellone, KCA’s Commercial Director, and discuss the matter of delta-8-THC, CBD conversion products in general and get the facts.
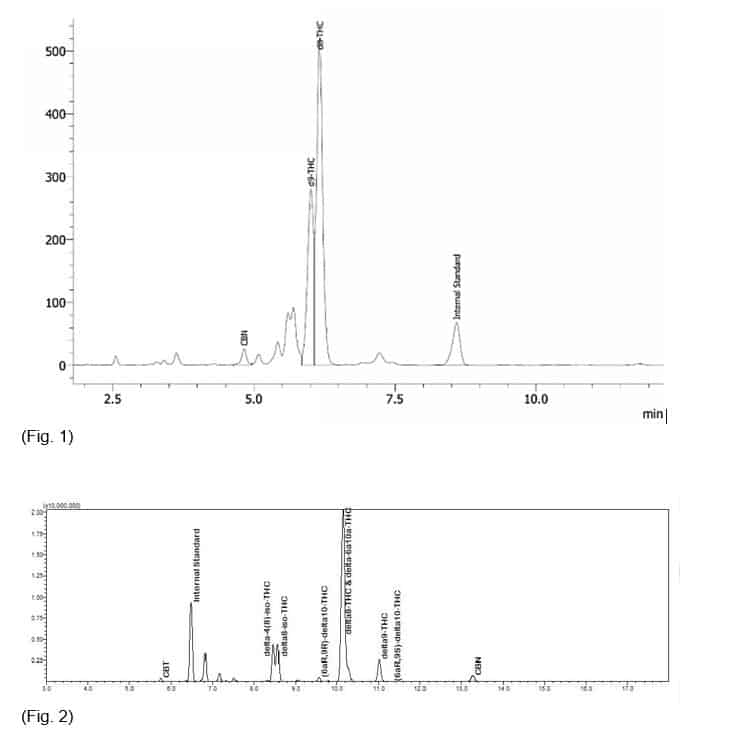
I arrived at the KCA Lab facility, where Dr. Sams and Ryan were very gracious and welcoming hosts, giving me a tour of the impressive state-of-the-art facility. I was impressed with their lab and the fact that all testing was performed by analysts with at least a four-year science degree. All their personnel who are responsible for issuing reports also have years of experience in performing compliance testing in various industries. A conversation quickly began at one of the mass spectrometry analysis stations about CBD conversion byproducts and testing issues. Dr. Sams asked one the chemists operating the nearby HPLC unit to open a sample analysis file for a recent delta-8 sample. A familiar graph appeared on the screen, typical of what I’ve seen so many times before (see Figure 1).
“See that group of spikes on the graph,” he motioned to a tight group of three or four spikes.
“I do,” I responded. “No one knows what they are, right?”
“Oh no, no. We know,” Dr. Sams said with a grin. “Not from this result, but from GC-MS (Gas Chromatography-Mass Spectrometry). Using liquid chromatography with photodiode array (PDA) detection system, you don’t get the specificity of mass spectral identification and the chromatographic resolution you get on GC-MS, and it tells you nothing about the chemical structure of these substances, yet it’s what’s used for most testing.”
Dr. Sams then pointed to the delta-8 spike on the HPLC graph and said, “Here, you see the peak for Delta-8. If you look closely, you can see what appears to be delta-9 on the leading edge of the peak, and it overlaps somewhat with the delta-8.” Indeed, the two peaks were overlapping. This makes accurate quantification of both the delta-8 and delta-9 in the sample impossible. The implication was clear: RP-HPLC-PDA (reversed-phase high performance liquid chromatography-with photodiode array detection) should not be used in compliance testing of CBD conversion products. There simply isn’t enough resolution between peaks to be useful in any meaningful way because CBD conversion products contain side-products that interfere with the detection of both delta-8 and delta-9 by RP-HPLC methods. Consequently, the delta-9 peak is a mixture of two or more substances and the delta-9 cannot be determined. In some cases, the impurity peak is identified as an impurity and not as delta-9 because it elutes closer to delta-8 than delta-9 does. Therefore, a sample could be interpreted as having little to no detectable delta-9, at all, when in truth, the product contains delta-9 above the limit. Furthermore, consumers using these products as a legal alternative to delta-9 are being misled. But just how likely would a situation like that be? It is difficult to say, but it could happen. For instance, consider a consumer who purchases flower that has been sprayed with delta-8 and sold as a legal product, meaning it tested as having less than 0.3 % delta-9-THC content, by weight. If that consumer is stopped by law enforcement after buying the flower, the product is confiscated and tested at the state level. The state utilized GC-MS and the result was the product test results showed it to contain 1.50% delta-9-THC. The hapless consumer is then charged with possession of a Schedule One controlled substance, due to no fault of their own, and the product manufacturer is shut down and mired in legal battles. Unlikely, but certainly possible.
Dr. Sams and Ryan led me to a GC-MS analysis unit and opened a data file containing the results of the same sample they had just shown me on the HPLC unit, and it looked completely different, with lots of separation between the critical peaks.
“Now look at the delta-8 and delta-9 peaks,” Dr. Sams said. There was a very definitive and wide separation between them (see Figure 2). “You can drive a truck through that space. These results are clearly not compliant when examined by GC-MS.” Indeed, it was obvious that this sample was non-compliant, and that’s a rather large problem, since virtually all industry cannabinoid profile and compliance testing is performed with HPLC.
When I asked if this was a common result, Ryan Bellone responded, “Very little of what is being produced is actually compliant, but it passes for compliant because testing is inadequate in most laboratories.” Ryan explained that when KCA tests a customer’s sample with GC-MS, and then reports that test result to the requesting lab customer, the customer is sometimes upset to learn their product is not compliant and “hot” with delta-9. The customer will sometimes even insist that KCA Labs must have made a mistake, since no other labs are getting the same result The reason for the discrepancy is that KCA Labs uses a much more specific method (GC-MS) that separated delta-9 from the side-products from the conversion of CBD.
When I asked if other analysis labs are aware of this issue, Ryan smiled and explained that many are, but if everyone suddenly switched to GC-MS for testing delta-8 for compliance, they’d lose customers because very few CBD conversions to delta-8 would be found compliant. So, it’s a slick little industry method to give the appearance of compliance. A processor could ask for GC-MS testing, but knowing the result would likely show an illegal concentration of delta-9, who would want to opt for GC-MS analysis? This isn’t an issue known to all processors because compliance testing labs aren’t likely to bring it up.
While compliance is an important legal issue, another issue getting a lot of press: product contamination from unwanted conversion byproducts. During the conversion process, different byproducts are created, some of which have been identified and some of which have not – substances that contribute to what appears to be delta-8-THC peak, yet they are not delta-8-THC. They’re similar in structure and lipophilicity, though, so they elute at almost the same times as delta-8-THC in when analyzed by HPLC, and that means they contribute to the delta-8 peak, hidden within it, unseen.
In the conversion sector of our industry, delta-8-THC is synthesized from cannabidiol under acidic conditions. Cannabidiol is exposed to a Lewis acid of one form or another, under certain conditions and the result is the intra-molecular ring closure of cannabidiol to form delta-9 which in then converted into delta-8-THC under these conditions. If this reaction does not go to completion, greater amounts of delta-9 will result. Then the reaction mixture is washed with bicarbonate in water to remove the acid catalyst. However, the conversion of cannabidiol also proceeds along another parallel pathway that produces those side-products that interfere with the HPLC analysis, but with GC-MS technique a completely different story emerges.
The two identified substances are delta-8-iso-THC and delta-4(8)-iso-THC. Dr. Sams and his team were successfully able to identify these substances via GC-MS analysis. Their contribution to what appears to be delta-8-THC is not insubstantial. Since the lipophilicities of the side-products are close to that of pure D8-THC, they cannot be readily separated and remain part of the finished product. Attempts to separate d-8-iso-THC and delta-4(8)-THC from delta-8 through chromatographic remediation would likely be futile or impractical. So, they’re in the finished product, but what does this mean to the consumer?
I asked, “What do they do? Are they safe, psychoactive and are they processed out of the body in the same manner as delta-8?”
“That’s the issue,” Dr Sams answered. “No one knows what these two substances might be doing in the body, what types of receptors they are binding to, specifically, or if they are harmful.” After discussing it further, we were all three in agreement that pure delta-8-THC, itself, is likely very safe, but no one can definitively say that yet about delta-8-iso-THC nor delta-4(8)-iso-THC and those substances are potentially present in all produced delta-8, as evidenced by the chromatograms obtained by GC-MS analysis.
Few labs and producers are aware of delta-8-iso-THC and delta-4(8)-iso-THC. Compliance testing does not point them out, HPLC is useless for identifying them and they appear to be delta-8-THC in most HPLC chromatograms. Currently, there isn’t enough data to reach any scientific conclusion pertaining to their safety and that’s a very real concern.
Dr Sams and Ryan went to the whiteboard and drew the molecules of delta-8-iso-THC and delta-4(8)-iso-THC, pointing out the differences between them and delta-8-THC, explaining that these differences were not minor ones. In the pharmacological industry, for instance, differences like these have often substantial consequences in the performance of a medication. They matter a lot. Everything from a medication’s efficacy to its side-effects can change, due to what might seem like minor structural differences between substances. I can cite the differences in the psychoactive properties of delta-8 and delta-9 as an example. In this case, the only molecular difference between the two substances is in the position of a single double bond in one cyclohexene ring of the molecule. That one tiny change results in a significant difference in how the product affects the consumer, though. After looking at the GC-MS results and my conversation with Dr. Sams and Ryan Bellone, I now question whether the difference felt is solely the result of that change in position of the double bond, or does the presence of delta-8-iso-THC and delta-4(8)-iso-THC modify the effect of delta-8?
On the drive home, my brain was reeling. Maybe D8-iso-THC and D4(8)-iso-THC are beneficial, somehow, but maybe they are not. Questions remain, concerning CBD conversion products, questions in need of definitive, scientifically-driven answers. Potentially every conversion isomer, be it delta-8, delta-10, HHC or any other, has these same types of issues. If psychoactive CBD isomers are to stay legal, the industry needs to tackle issues and find ways of removing all undesirable substances or finding conversion acids that don’t create those byproducts – if that’s even possible. Until the industry standard analysis moves to GC-MS, no one will know exactly what they are producing and selling, and that’s a big problem.