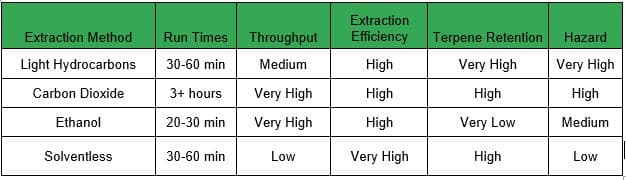
There are a lot of options for extraction – all of which have benefits and drawbacks – and making the right choice depends on the situation and starting material.
We have been working in cannabis and pharmaceutical manufacturing for over two decades. We are often asked for opinions about the science involved. Probably the most common inquiry we get is, “which extraction method is best?” It’s a great question with many right answers depending on the material and end product desired. With that in mind, we’d like to break down four of the more common extraction techniques and talk about the pluses and minuses associated with each one of them.
Before we start, we want to address safety. Simply put, some methods can be quite dangerous because of the chemicals involved. They should only be done with adequate process and engineering controls. Additionally, having the correct education and experience is critical for safe operations. Extraction isn’t worth it if you are putting yourself or your employees at risk. The good news is that there are options that are much safer than others.
Light Hydrocarbon
Hydrocarbons are extremely volatile organic chemical compounds which contain only carbon and hydrogen atoms. The two most commonly used hydrocarbons in cannabis extraction are propane and n-butane. These hydrocarbons are single-bonded and are classified as non-polar alkanes because their electrical charge is evenly distributed across the molecule. This makes them an ideal extraction solvent because cannabinoids and terpenes are also nonpolar, and in chemistry “like dissolves like.”
Benefits: Very non-polar solvent that has a high affinity for terpenes and cannabinoids. With typical batches of 5-10 lbs and a run time of 30-60 minutes you can produce up to 8 kg (~18 lbs) of concentrate a day. This is the only solvent-based extraction method that is compatible with small amounts of water, which allows the extractor to run “fresh frozen” biomass. These runs produce live resin concentrates that contain a true-to-cultivar taste that is considered by many to be unmatched by other extraction methods.
Drawbacks: Light hydrocarbons are a gaseous solvent at 30°F and are the most volatile and combustible solvents used in cannabis extraction. These gases are denser than air and sink to the floor, so strict airflow protocols and emergency procedures are necessary. Propane and n-butane also have a very low explosive limit: the concentration of gas in the air only has to reach between 1.8% and 8.4% to create dangerous conditions. Because of this hazard, all hydrocarbon extractions and secondary processes must take place in a Class 1 Division 1 (C1D1) lab space.
Ethanol is a liquid at room temperature and ambient atmospheric pressure, and is highly polar due to the presence of the hydroxyl group (the oxygen and hydrogen cluster). The highly negative electrical charge of the oxygen allows the hydrogen to more easily bond with other polar molecules. However, the ethyl group is nonpolar, so it attracts nonpolar molecules. Extractors can “lock out” the polar properties of ethanol by chilling the solution to below -20°C which makes for a relatively safe non-polar extraction solvent.
Benefits: Ethanol extractions have a very high throughput due to their non-polar properties, simplicity of the extraction method, and the ability to run over 100 lbs per batch in less than 30 minutes. With ethanol, you will be able to quickly produce a lot of crude oil with minimal post-processing. This is a great solution for a processor who wants to make full-spectrum cannabis oil, edibles, distillate, or isolate. Crude can be refined to extremely high purity levels, with some producers capable of creating 98% pure THC or better.
Drawbacks: The biggest drawback for ethanol extraction is that it does not effectively preserve the terpene profile of the plant, which leads to a concentrate that is very homogenous across all biomass. In more basic terms, dabbing ethanol concentrates is generally not pleasant for consumers. You need large amounts of solvent to perform ethanol extraction, and a large-scale solvent recovery system. Storage of ethanol requires a firm understanding of and adherence to the National Fire Code, and many local governments require a Class 1 Division 2 (C1D2) environment for an ethanol lab.
Carbon Dioxide Extraction
Carbon dioxide (CO2) is a non-polar solvent that is gaseous at ambient room temperature and pressure. However, when CO2 is placed under pressure, it undergoes a phase transition and is transformed into either a supercritical or subcritical fluid that can be used for cannabinoid and terpene extraction.
Benefits: CO2 aggressively strips away the desirable cannabinoids from the plant material. The cannabinoid-rich solution is then moved through the extraction system to a separator, where the desirable compounds are collected. Due to the gaseous nature of CO 2 at room temperature, the concentrates produced from the system are virtually free of residual solvent. However, the cannabinoid extract that remains is still heavily saturated in the plant’s fats and waxes. This makes CO2 extraction ideal for creating “full spectrum” products, but means they also require additional post-processing to produce a market-ready product unless inline winterization is an option. CO2 is perhaps the easiest, and safest, method for solvent extraction. It also scales well, and could easily be used to extract tons per day instead of pounds per day.
Drawbacks: Even though CO2 is not flammable or combustible, the extraction systems used in the process are not completely hazard-free. A CO2 system is frequently placed under immense pressure, sometimes as high as 2500 psi (~172 bar); if the equipment is not properly maintained, there is the potential for a pressure explosion. Also, because CO2 is odorless and colorless, it is imperative to monitor for leaks constantly. CO2 is an asphyxiant, which means you could die of suffocation if the concentration of the gas within the lab becomes too high.
Solventless Extraction
There are two commercially viable methods of solventless extraction: ice water extraction and rosin pressing.
Ice water extraction is where biomass is submerged in cold water, causing the trichomes to become brittle. The plant material is then agitated with a mixing spoon and ice to physically knock the trichomes off of the biomass. Ice water hash is easy to smoke, and when made well, is very delicious and clean.
Rosin pressing is where biomass or hash is measured into a micron screen bag then put between two heated plates. These plates are then pressed together to squeeze the cannabinoids and terpenes out through a channel, leaving the undesirable material behind on the plates.
Benefits: Unlike solvent methods, there is no need to purge the extract after the initial extraction, and there is no need for extensive post-processing; basically, the material just has to be left to dry and cure, and perhaps pressed into a solid form. It is by far the safest approach to extraction.
Drawbacks: These methods are relatively time consuming and tend to have very small batches. This leads to issues with throughput and an increased cost to manufacture. Ice water extractions have become more scalable with new technology, but rosin pressing has a very finite capacity.
As you can see, there are four distinct approaches used by most cannabis extraction companies. All of them have significant benefits, but also significant risks. Make sure that you choose one that will protect your safety and the safety of your employees and contractors. That should always be your first consideration. Once you have ensured that you are properly addressing issues related to danger, start by determining what you want the end product to be and pick a method that will help you get there. Happy extracting!
Duke Fu is Chief Operating Officer at Australis Capital (AUSA) and was previously CEO of Green Therapeutics. He holds MBA and Doctor of Pharmacy degrees from the University of New Mexico and is a board-certified nuclear pharmacist in the state of Nevada, where he resides.
Anthony DeMeo is co-chair of Commercial Extraction and Manufacturing at Oaksterdam University and was a founding member of Green Therapeutics. He has extensive experience in cannabis extraction methods, including various solvents, ice water, and mechanical separation, and has also developed and optimized post-processing techniques to produce high quality concentrates, distillate, and isolates.