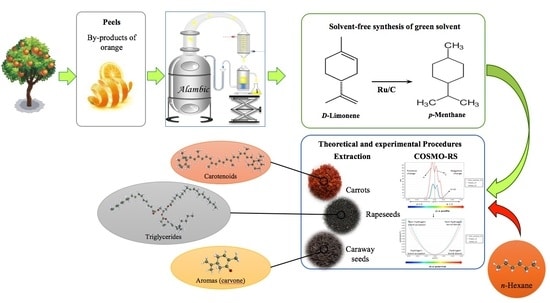
Terpenes have been explored as green solvents with the potential to replace petroleum-based solvents such as n-hexane. Limonene is a terpene solvent of great interest. It is a common waste product (e.g., citrus peels) and has illustrated success when extracting oil from rapeseed and microalgae as well as carotenoids from tomatoes, among others. [1] That said, Madji et al [1] note that limonene “oxidizes easily under exposure to air,” and “oxidation products are labelled as allergens.” Given these limitations, the researchers determined to hydrogenate limonene to synthesize a more stable yet still effective terpene solvent: para-menthane.
Initially, they conducted a theoretical study using specialized software to predict solubility of certain target compounds in n-hexane or p-menthane. The commonly extracted foods and targets were:
- Carotenoids from carrots
- Triglycerides from rapeseeds
- Terpenes D-limonene and S-carvone from caraway
The researchers noted that “p-menthane [had] probability of solubility higher than, or similar to, n-hexane.” The experimental part of the study then tested each solvent’s ability to extract the listed compounds from their respective food sources.
Reprinted from: Madji S, Hilali S, Fabiano-Tixier AS, et al. para-Menthane as a stable terpene derived from orange by-products as a novel solvent for green extraction and solubilization of natural substances. Molecules. 2019;24(11):2170. doi:10.3390/molecules24112170. License: Attribution 4.0 International (CC By 4.0)
The limonene came from essential oil of citrus peel waste. A relatively low temperature (40-50° C) and ruthenium catalyst on activated charcoal were used to hydrogenate limonene to p-menthane. The main extractions relied on the ULTRA-TURRAX® Tube Drive System with 1 g ground plant material (carrot, rapeseeds, or caraway) and 10 ml solvent (n-hexane or p-menthane) for solid-liquid extraction.
The solvents yielded similar quantities of α- and β-carotene from carrots although the mass yield from p-menthane was slightly higher (not significantly). The triglyceride profiles of the rapeseed oil extractions were nearly identical. Results for caraway’s key terpenes, D-limonene and S-carvone, were similar although n-hexane produced slightly higher (not significantly) yields. Thus, the solvents were comparable with no significant differences.
To further investigate p-menthane, the researchers conducted two additional “common analytical extraction procedures”: Dean-Stark Distillation (separating water from plant matrices) for carotenoids from carrots and Soxhlet extraction for oil from rapeseeds. These experiments compared p-menthane to toluene rather than n-hexane. Again, results between solvents were very similar. The only significant difference regarded Dean-Stark Distillation, where complete water recovery with p-menthane required 55% less time compared to water recovery with toluene. This improvement came after a brief starting delay due to p-menthane’s higher boiling point.
Ultimately, the authors declare, p-menthane could be a “green replacer for petroleum-based solvents such as n-hexane or toluene.” [1]
Reference
- Madji S, et al. para-Menthane as a stable terpene derived from orange by-products as a novel solvent for green extraction and solubilization of natural sMolecules. 2019;24(11):2170. doi:10.3390/molecules24112170. [Impact Factor: 3.267; Times Cited: 1 (Research Gate)]