Terpenes are a common target of botanical extraction; they are also valuable byproducts re-introduced into concentrates. But can terpenes act as solvents?
Terpenes may be a boon on the quest for alternative green solvents. They are hydrocarbon molecules (C10H16)* with properties that Boutekedjiret et al [1] suggest make them viable as “substitutions of petrochemical solvents” such as n-hexane (C10H14). These attributes are known as Hansen solubility parameters. They map how well a solvent will dissolve a material. As it happens, the solubility parameters for monoterpenes are similar to n-hexane. These parameters are:
- Energy from dispersion forces (𝛿d)
- Energy from dipolar intermolecular forces (𝛿p)
- Energy from hydrogen bonds (𝛿h)
These properties help explain the use of terpenes like cymene in dyes and varnishes. [2] As another example, limonene is a common commercial/industrial cleaning solvent. More relevantly, α-pinene, the principal component of turpentine and a monoterpene common to cannabis, has been found to produce higher yields of olive, sunflower, and peanut oils when replacing n-hexane in Soxhlet extraction. [1] The yield difference was likely due to higher polarity. [1] Carotenoids—orange pigment molecules with antioxidant properties (carrots, anyone?)—are also a viable target for α-pinene and limonene. [1]
Recently, terpenes have been explored as key components for natural deep eutectic solvents (DESs)—monoterpenoid alcohol menthol, in particular, may be combined with certain acids to extract cannabinoids from hemp. The principle of natural DSEs is to use a terpene as a hydrogen bond accepter (HBA) in combination with a hydrogen bond donor (HBD) to create strong hydrogen bonding. Note that hydrogen bonding relates directly to solubility parameters (see above).
Martins et al [2] cite “unsustainable consumption of nonrenewable and environmentally questionable chemicals,” as a key impetus for terpenes as solvents. This group of researchers created binary mixtures of different terpenoids to see if combinations could allow extractors to fine-tune the use of DESs. The tested terpenoids included menthol, thymol, camphor, borneol, and trans-sobrerol. Thymol affected the mixtures significantly due to an “acidic proton” on its hydroxyl (OH) group; menthol, with a higher capacity to accept bonding, interacted strongly with thymol. Camphor was the least likely terpene to act as a donor. Overall, physicochemical properties could be modified by mixing terpenoids. The authors concluded that “extraction and selectivity can be manipulated by varying the mole fraction of the terpenes in the mixture.” [2]
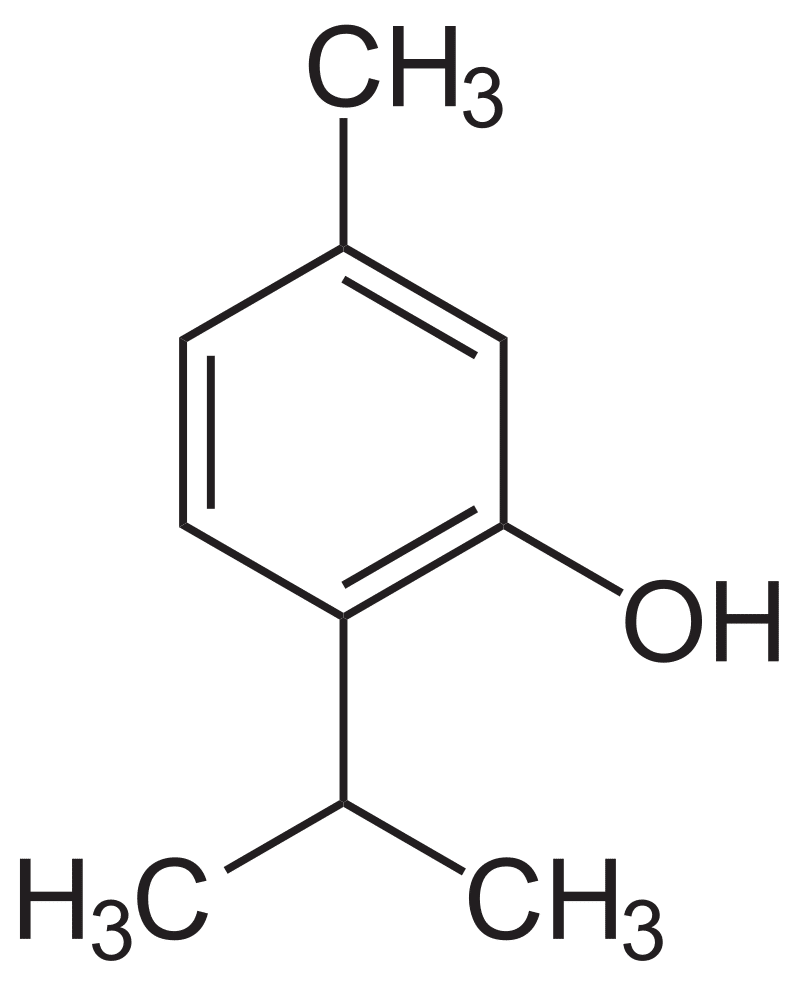
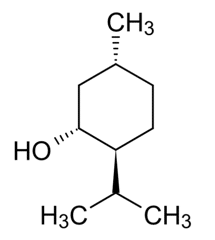
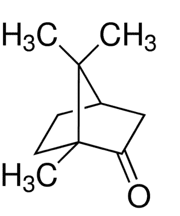
Thymol. Public Domain
Menthol. Public Domain.
Camphor. Claudio Pistilli—CC By-SA 4.0.
Studies investigating the use of terpenes as primary solvents for cannabis extracts have not yet been conducted (other than the DESs used to isolate hemp cannabinoids linked above). However, given their natural properties, terpenes may generate a new, all-natural, ‘green’ style of concentrate. It’s entirely plausible that garage extractors and private producers have already started experimenting.
References
- Boutekedjiret C, et al. Chapter 9: Terpenes as green solvents for natural products extraction. In: Chemat F, Vian MA, eds. Alternative Solvents for Natural Products Extraction. Green Chemistry and Sustainable Technology. Berlin: Springer; 2014. https://doi.org/10.1007/978-3-662-43628-8_9. [Times Cited (chapter): 5]
- Martins MAR, et al. Greener terpene-terpene eutectic mixtures as hydrophobic solvents. ACS Sustainable Chemistry & Engineering. 2019;7(20):17414–17423.doi:10.1021/acssuschemeng.9b04614. [Times Cited: 3; Impact Factor: 7.632]
Image: Gerd Altmann from Pixabay
*Note: Two isoprene units (C5H8) form the monoterpene