Analytical testing is an extremely important aspect of the cannabis industry. In many ways, it’s what sets the industry apart from the black market—the fact that when people go to a dispensary, they are able to look at a label that shows the exact cannabinoid (and, ideally, terpene) content of a concentrate or flower, and that the products being on the shelves in the first place should mean they have passed safety testing for pesticides levels, residual solvents, and other nasty contaminants we wouldn’t want to ingest!
A cannabis testing facility will have several methods on hand to determine each of these given parameters. Among the most popular is gas-chromatography/mass-spectrometry (GCMS), which vaporizes small samples and analyzes the resulting molecular composition of the gases. Another popular method of analysis is high-performance liquid chromatography (HPLC), which separates compounds based on the length of time that they interact with a stationary phase of functionalized glass beads using a specific mobile phase or solvent(s), and analyzes the resulting outflow (eluent) with different detection techniques to determine the relative percent (or qualitative presence) of which molecules abound.
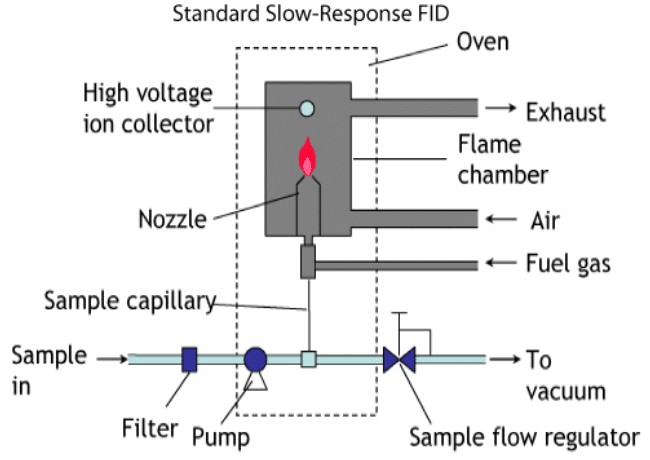
Flame Ionization Detection, or FID (pronouncing each letter), is a cheap and standard detector which has a long history of use in the automotive industry for measuring the total hydrocarbon concentration, as well as various other molecular species, in a given engine emissions sample. In modern application, the FID can detect many different types of molecules, including complex hydrocarbons and oxygenated species—in other words, cannabinoids and terpenes. The FID is usually coupled to a gas chromatograph, which serves to vaporize and separate the components of the sample before they reach the FID. See an example of what an FID setup looks like in the figure below:
What the FID does in practice is continuously keep alight a flame of pure hydrogen gas in a chamber filled with air. Since carbon dioxide is not detected by this procedure, regular room air can be used, as opposed to pure oxygen. As the sample gas is fed across the flame, any hydrocarbons that are present combust. Some of these combustion events produce carbon ions, which are then detected by an electric component inside the chamber. The number of ions produced, and therefore the electric signal, are proportional to the amount of hydrocarbons that were present during that particular ejection time of the gas chromatograph.
Together, with additional analysis and by comparing to standard literature data, the data produced by a GC-FID can confirm with a high degree of accuracy the amount of a specific cannabinoid or terpene that the sample has present. [1] What the FID is extremely sensitive for, however, even more than the large and bulky cannabinoids and terpenes, are residual solvents. Propane, butane, hexane, even alcohols and acetone, are all easily detectable by FID even at extremely low (10-13, or 0.1 parts per trillion) levels. This makes it an ideal instrument to include in your testing repertoire as the popularity of solvent-based extracts continue to grow.
References
- Giese, Matthew W. et al. “Method for the Analysis of Cannabinoids and Terpenes in Cannabis”. Journal of AOAC International. 2015; 98(6): 1503-1522 [Times cited = 26, Journal impact factor = 1.120].
Image Credit: Wikimedia Commons