Cannabis Sativa plants produce and accumulate terpene-rich resin within the secretory cells of glandular trichomes. Monoterpenes and sesquiterpenes are important components of Cannabis resin as they contribute to the unique attributes of different Cannabis strains. Solventless terpene extraction is responsible for the plant’s aroma and flavor.
Unlocking the Therapeutic Potential of Terpenes and Cannabinoids
Terpenes, the aromatic compounds found in various plants, have gained increasing attention for their potential medical properties. When it comes to Cannabis, these terpenes not only offer unique flavors and scents but also have specific medical properties that may synergize with cannabinoids, enhancing the therapeutic benefits of the plant. The extraction of terpenes and cannabinoids from Cannabis is a critical process, heavily dependent on their solubility in different organic solvents.
Common solvents like methanol, ethanol, butane, and hexane are often used for Cannabis extractions. However, the drawback of using these solvents is their lack of selectivity; they produce extracts with a “one pot extraction” approach, making it impossible to distinguish between cannabinoids and terpenes. In this article, we delve into the challenges of terpene extraction and explore the use of supercritical fluids as a promising solution for isolating terpenes from cannabinoids in Cannabis.
The Dilemma of Solventless Terpene Extraction
Discussing solventless terpene extraction presents a challenge on two fronts. First, during post-processing, thermally labile terpenes can undergo degradation reactions, leading to a loss of their medicinal properties. Secondly, the isolation of terpenes from conventional solvents poses difficulties due to their similar boiling points. These issues have raised concerns about the efficacy and safety of traditional extraction methods, necessitating a closer look at alternative approaches. The delicate nature of terpenes and their significance in the “entourage effect” have sparked interest in finding more selective extraction techniques that preserve their integrity.
The Entourage Effect and Promising Applications
The interaction between cannabinoids and terpenes in Cannabis strains is a subject of great intrigue, often referred to as the “entourage effect.” Emerging data suggests that this synergy between different compounds affects the pharmacological properties of Cannabis. This phenomenon opens the door to several promising applications in the field of medicine. For instance, combining cannabidiol (CBD) with specific monoterpenes like limonene, linalool, and pinene shows potential in treating conditions like acne.
Additionally, augmenting 1:1 CBD/THC extracts with caryophyllene, linalool, and myrcene demonstrates promise in addressing sleeping disorders. Understanding the intricate relationships between cannabinoids and terpenes is crucial for harnessing their therapeutic potential effectively.
 Terpene Classification and Biosynthesis
Terpene Classification and Biosynthesis
Terpenes encompass a diverse group of compounds, with their classification based on the number of isoprene units they contain. Monoterpenes, the smallest and most volatile of terpenes, are synthesized through the head-to-tail addition of two isoprene units (Figure 1). Each isoprene unit consists of five carbon atoms, forming the foundation upon which terpenes are built.
Combining three isoprene units leads to the creation of sesquiterpenes, which are less volatile than their monoterpenes counterparts. Finally, the largest and least volatile terpenes are formed by joining four or more isoprene units, offering a rich diversity of compounds with varying properties.
Chemical Transformations of Terpenes
Terpenes are fascinating molecules known for their volatility and susceptibility to various chemical transformations. These transformations can include oxidation, isomerization, cyclization, or dehydrogenation reactions (Figure 2). The environment in which Cannabis is stored and the methods used in its processing can profoundly impact the chemical composition of these isoprene-containing compounds.
For example, the distillation of crude Cannabis oil presents two significant challenges. First, the high temperatures required for distillation can lead to the thermal degradation of delicate monoterpenes. Second, during distillation, organic solvents can co-elute with the terpene fraction. The presence of these organic solvents in terpene fractions can lead to contamination in the final Cannabis oil product.
Supercritical Fluid Extraction (SFE) with CO2
Supercritical fluid extraction (SFE) has emerged as an effective method for separating monoterpenes from sesquiterpenes, their alcohol derivatives, and cannabinoids. Among the various supercritical solvents, carbon dioxide (CO2) has gained popularity as the preferred choice. CO2 is not only cost-effective but is also generally recognized as a safe solvent. It reaches a supercritical state at specific conditions—31°C and 74 bar—and returns to a gaseous state at ambient conditions. This unique property allows for the simple recovery of solutes and results in solvent-free extracts.
Moreover, by fine-tuning the pressure and temperature of the CO2 system, one can tailor the solvent’s dissolution properties, achieving a selective extraction of cannabinoids and terpenes. In the sections that follow, we will outline a systematic approach to developing optimal solventless terpene extraction conditions utilizing supercritical carbon dioxide.
Experiment 1
In the quest to develop precise conditions for solventless terpene extraction using supercritical carbon dioxide (SC-CO2), understanding the relative solubility of terpenes becomes a critical endeavor. While solubility data for cannabinoids in SC-CO2 is well-documented, information regarding the solubility of terpenes in this medium remains limited. In this article, we delve into Experiment 1, where we systematically investigate the elution order of monoterpenes and their separation from sesquiterpenes under various SC-CO2 conditions.
Transforming the Investigator SFC System
To carry out this study, we employed the Investigator Supercritical Fluid Chromatography (SFC) instrument, a versatile tool designed primarily for analytical and semi-preparative purification work. With some minor modifications, this SFC unit was adeptly transformed into a benchtop preparative Supercritical Fluid Extraction (SFE) system, perfectly suited for our investigative needs.
Experimental Setup and Procedure
Our experimental setup involved acquiring a 20 mL SFC column and carefully removing its column packing, effectively converting it into an extraction vessel tailored for benchtop SFE experiments. We installed a three-way valve before the photodiode array detector and plumbed 1/16-inch tubing directly into the backpressure regulator, bypassing the detector. The Fraction Collection Module was disconnected to collect fractions enriched with our target compounds as they exited the automatic backpressure regulator (ABPR) and were deposited into scintillation vials.
Column Packing and Sample Injection
The SFC column was pre-packed with diatomaceous earth, and we prepared a solution containing equal molar amounts of α-pinene, β-myrcene, d-limonene, linalool, and β-caryophyllene by sonicating it for 10 minutes. We then injected 400 µL of this solution into 0.5 g of diatomaceous earth, which was loaded into the head of the pre-packed SFC column. The system was equilibrated to 50 bar, with the oven temperature set at 30°C, maintaining a constant flow rate of 1 mL/min throughout the extraction process.
Fraction Collection and Analysis
During the 42-minute experiment employing pure CO2 as the mobile phase, we collected a total of twenty-four 15 µL fractions. Terpene elution commenced at the 10-minute mark, with fractions 1 through 11 being collected between minutes 10 and 14. Subsequently, the elution of terpenes temporarily ceased until minute 36, when it resumed. Fractions 12 through 24 were then collected between minutes 36 and 42. Each fraction underwent analysis using Gas Chromatography with Flame Ionization Detection (GC-FID), recording peak heights for terpenes.
Data Analysis and Solubility Determination
Linalool and beta-caryophyllene were consistently observed in each fraction, with linalool chosen as the normalization peak due to its highest signal production. To standardize the data, the peak height of each terpene was divided by the peak height of linalool. In Table 1, we highlight the terpene fractions with the highest terpene-to-linalool ratio. By comparing these ratios, we can ascertain the elution behavior of terpenes under specific SC-CO2 conditions.
Terpene Solubility Findings
Our findings revealed distinct solubility patterns. Alpha-pinene, beta-myrcene, and d-limonene exhibited their highest concentration in fraction 2, all eluting consistently at 50 bar and 30°C. The ratio for beta-caryophyllene exhibited a turning point in fraction 12, with a ratio exceeding 1, indicating a higher concentration of beta-caryophyllene than linalool. This data suggests that the solubility order of these terpenes at the specified SC-CO2 conditions is as follows: α-pinene, β-myrcene, d-limonene, linalool, and β-caryophyllene.
Experiment 2
Next, the conditions from Experiment 1 were used to extract 5 grams of Cannabis inflorescence (the mature flower of a female plant). A similar procedure was followed: this time, a 5 mL SFC column was obtained, and the packing was removed. Five grams of cannabis inflorescence were ground, packed into the 5mL SFC column, and installed into the Investigator’s oven.
The Investigator system was equilibrated to 50 bar, and the oven was set to 30°C. The flow rate was maintained at 7 mL/min. No fractions were observed in the first 60 minutes, at which time the system was equilibrated to 100 bar, maintaining temperature and flow rate. Three fractions were collected, with the elution starting at 80 minutes. These fractions were analyzed using GC-FID and compared with the terpene quantification of the starting material. The results were not satisfactory for two reasons:
- In this first attempt, we were unable to recover our volatile terpenes;
- The length of extraction was prohibitively long.
If these conditions were scaled to a 5 L system (vs our 5 mL system), the solventless terpene extraction would take over three hours, requiring a significant amount of CO2. This experiment was repeated multiple times, modifying both the instrumentation and supercritical carbon dioxide conditions until both monoterpenes and sesquiterpenes were recovered in an appropriate length of time. The next section will examine the modifications and conditions required to scale up to a 5-litre SFE system.
Supercritical Extraction Equipment and Terpene Extraction Procedure
Scaling up solventless terpene extraction requires specialized equipment capable of handling larger volumes and maintaining precise conditions. In this endeavor, we employed a 5 L Bio-Botanical Extraction System (Waters, Milford, USA), which underwent modifications to enhance its capabilities.
Notably, a fourth terpene collection vessel (CS4) was integrated into the system to accommodate the expanded scale of extraction. This setup boasted a maximum operating pressure of 600 bar, with extraction pressure regulated by the Automatic Backpressure Regulator (ABPR). Temperature control within the extraction vessel was managed by an electrical jacket. The system featured three collection vessels (CS1-CS3), each independently heated and equipped with manual pressure control. The high-pressure pump ensured a maximum mass flow rate of 200 g/min.
Supercritical Carbon Dioxide Extraction Process
The essence of supercritical carbon dioxide (SC-CO2) extraction lies in its ability to selectively dissolve target components from botanical materials. To achieve this, the solvent, CO2 in its supercritical state, is directed through a heat exchanger, bringing the liquid CO2 to the required supercritical state. The supercritical stream carries the target compounds away from the extraction vessel to designated cyclone separators, where they can be collected for further processing. Table 2 summarizes the optimized conditions used for this extraction process.
 Targeting Cannabinoids and Terpenes
Targeting Cannabinoids and Terpenes
Cannabinoids and terpenes, while both soluble in supercritical carbon dioxide, exhibit varying solubility profiles. Additionally, compounds within these classes display unique solubility characteristics. In the extraction of terpene-rich hemp, we adopted a multi-stage approach, increasing the density profile of SC-CO2 at each stage to target different compounds effectively.
Stage 1: Monoterpene Extraction
Our initial stage focused on the extraction of volatile monoterpenes. To accomplish this, we directed the flow of supercritical carbon dioxide into CS4, effectively bypassing cyclone separators one through three. Without the inclusion of CS4, volatile monoterpenes tend to escape with the supercritical carbon dioxide, exiting from SC3 into the CO2 recycler. By introducing CS4, we created conditions conducive to the recovery of volatile monoterpenes.
However, even with CS4 in place, volatile monoterpenes would still escape at ambient temperatures along with the gaseous CO2. To counter this, CS4 was chilled to an astonishing -78°C using a solvent mixture of acetone and dry ice. Once adequately chilled, the extraction vessel was pressurized to 70 bar, and extraction commenced at 50°C. These conditions proved ideal for the high solubility of monoterpenes in supercritical carbon dioxide, while sesquiterpenes exhibited mild to low solubility.
Stage 2: Sesquiterpene Extraction
Moving on to Stage 2, our goal was to target less volatile sesquiterpenes. In this phase, the flow of supercritical carbon dioxide was redirected into CS1 and continued through CS3. After a duration of 45 minutes, sesquiterpenes were collected from CS3. This stage involved an increase in both temperature and pressure to effectively dissolve the targeted compounds. The collected terpene fractions from both Stage 1 and Stage 2 underwent analysis using Gas Chromatography with Flame Ionization Detection (GC-FID), with results depicted in Figure 3.
Stage 3: Cannabinoid Extraction
In the final stage of the extraction process, the pressure parameter was elevated to 300 bar to target cannabinoids effectively. Monoterpene extraction took 45 minutes, while cannabinoid extraction required an extended duration of 5.5 hours. This comprehensive approach yielded an impressive total efficiency of 92%, resulting in the extraction of a significant portion of the available target compounds. This stage represented the culmination of our efforts, successfully extracting both terpenes and cannabinoids, showcasing the efficacy of the scaled-up solventless terpene extraction process.
Conclusion
In order to develop solventless terpene extraction conditions utilizing supercritical carbon dioxide, it is essential to understand the relative solubility of terpenes in supercritical carbon dioxide. It was determined that monoterpenes have high solubility at 70 bar and 50°C while sesquiterpenes have low to mild solubility at these conditions. With the addition of a terpene-specific collector (CS4), supercritical CO2 is an effective solvent for the extraction of terpenes from Cannabis.
The ability of supercritical carbon dioxide to return to a gaseous state once exposed to ambient conditions allows for simple terpene recovery and results in no detectable residual solvents. Monoterpene fractions were obtained from the terpene specific collector with high purity and no detectable cannabinoids. In order to recover highly volatile monoterpenes, CS4 needed to be chilled to -78°C.
Plant waxes and cannabinoids co-elute with sesquiterpenes, which are collected from CS3. Due to the more robust nature of sesquiterpenes, these compounds can undergo post-processing methods such as winterization with minimal to no degradation. The ability to separate monoterpenes from sesquiterpenes from cannabinoids allows for the post-processing of cannabinoids without the danger of terpene degradation.
References
- Gershenzon J, McCaskill D, Rajaonarivony JIM, Mihaliak C, Karp F, Croteau R (1992) Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal Biochem 200:130–138.
- Perrotin-Brunel, H.; Perez, P. C.; van Roosmalen, M. J. E.; van Spronsen, J.; Witkamp, G. J.; Peters, C. J.Solubility of Delta(9)-tetrahydrocannabinol in supercritical carbon dioxide: Experiments and modeling J. Supercrit. Fluids 2010, 52, 6–10 DOI:10.1016/j.supflu.2009.12.001
- McPartland JM, Russo EB (2001). Cannabis and cannabis extracts: greater than the sum of their parts? J Cannabis Therapeutics 1: 103–132.
- Aizpurua-Olaizola O, Soydaner U, Ozturk E, Schibano D, Simsir Y, Navarro P, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemo-types. Journal of Natural Products. 2016; 97: 324–331.
- McGraw, G. W.; Hemingway, R. W.; Ingram, L. L.; Canady, C. S.; McGraw, W. B. Thermal degradation of terpenes: Camphene, Delta(3)-carene, limonene, and alpha- terpinene Environ. Sci. Technol. 1999, 33,4029– 4033 DOI: 10.1021/es9810641
- Rovetto, Laura & V. Aieta, Niccolo. (2017). Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. plant material. The Journal of Supercritical Fluids. . 10.1016/j.supflu.2017.03.014.
- Waters. “Investigator SFC System.” Power of analytical method development and small-Scale purification : Waters,

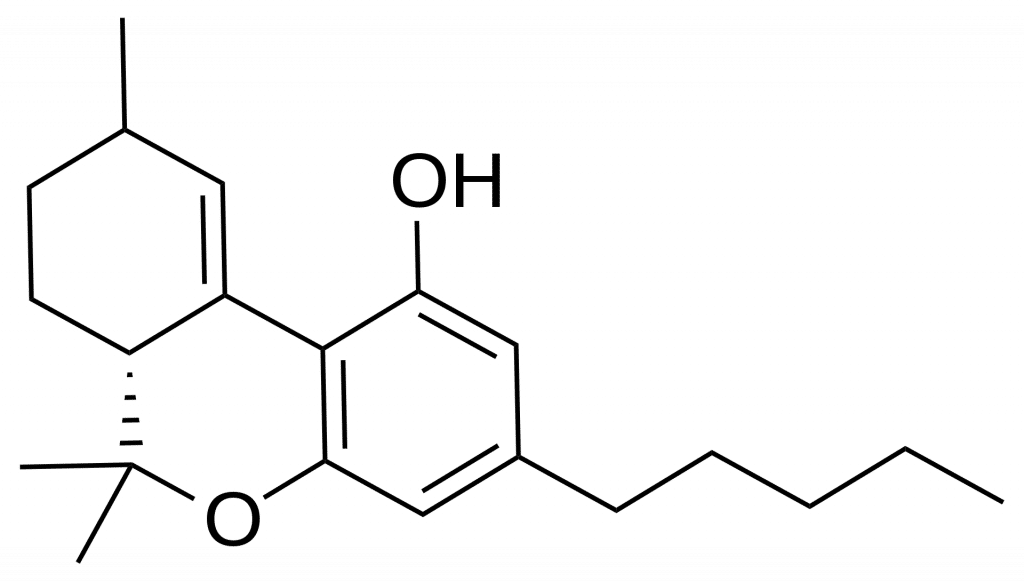 Terpene Classification and Biosynthesis
Terpene Classification and Biosynthesis Targeting Cannabinoids and Terpenes
Targeting Cannabinoids and Terpenes










