The importance of temperature differences at different stages of the BHO/CO2 closed loop.
Closed-loop systems, such as the extraction equipment that powers hydrocarbon and supercritical CO2 extractions, require the use (and variation) of pressure to achieve their desired ends. The intricacies of one system versus the other is something we discuss in depth elsewhere. In this article we would like to give a head nod towards the physical properties that both of these extraction systems take advantage of. The branch of science that addresses the interaction between physical things, or matter, and the physical conditions that matter resides in (such as temperature, pressure, and volume), is called Thermodynamics.
Robert Boyle published his observation in 1662 that, when holding the temperature of a gas constant, the pressure and the volume of a gas are inversely related to each other (as one goes up, the other goes down).The temperature of a gas in absolute temperature is directly proportional to the pressure in the container when the volume is held constant.This relationship is known as the Gay-Lussac law.
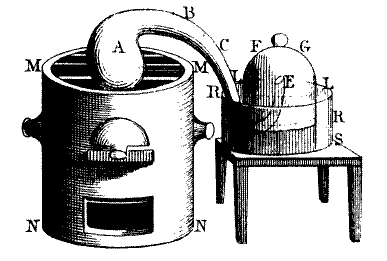
In many ways, the field of thermodynamics (and really, modern chemistry)dates back to 1774, when French scientist Antoine Lavoisier published his treatise, “TraitéÉlémentaire de Chimie”, or,the Elementary Treatise of Chemistry. In it, he detailed his experiments on combustion of materials in air and formulated the Law of Conservation of Mass. The headline image is a diagram of the sealed containers in which Lavoisier was able to establish that the materials gained in mass when burned in air.
Following the publication of Lavoisier’s work in the 18th century, scientists dedicated a lot of time to figuring out how gases work. Unlike solid samples, gases could not be studied directly under a microscope. Therefore, these scientists had to study gases indirectly through measurements. A great deal of research into gases was also performed by engineers who created the steam engine. Steam engines, which for almost two centuries powered trains, ships, and factories, were major driving forces of the Industrial Revolution.To make better engines, engineers and scientists strove to find the relationships that governed the mathematical relation between the pressure, temperature, and volume of a gas.
The Kinetic Theory of Gases forms the basis for explaining the macroscopic relationships seen in thermodynamics. Experimentally, it can be observed that in a closed container with a known volume of gas, as the temperature of the system drops, we observe the pressure drop in a linear fashion. William Lord Kelvin, a Scottish-born physicist and engineer, extrapolated the linear relationship to the point where the pressure of the gas equals zero. The theoretical temperature that he calculated was minus 273.15°C.Since all the energy of a gas is kinetic (based on movement), this is the point at which gas molecules have no energy,also referred to as absolute zero.
The correct way to think about kinetic theory is this: pressure, defined as the average force per unit area, is generated by the collisions of gas molecules against a surface. The temperature of a gas in absolute temperature is directly proportional to the pressure in the container when the volume is held constant. And both the pressure and the temperature are inversely related to the volume.
These observations together form the Ideal Gas Law, which is like the first commandment of thermodynamics. From here, all knowledge regarding how matter interacts with itself and its surroundings is derived. In the setting of cannabis extraction, temperature will determine the speed at which cannabinoids and terpenes are solvates, meaning how long solvent must immerse the plant matrix before desirable compounds are pulled out. Changing temperature is an indirect way to manipulate pressure, since the two are directly correlated. So, attaching a water heater to the collection tank of a hydrocarbon extractor will reduce the pressure and cause the evaporation of carrier solvent that can then be condensed and recycled inside of a chiller. In supercritical CO2, temperature and pressure play an even more important role, as their properties directly determine the hydrophobicity of the solvent itself.
So, take some time to appreciate what gases are, and it’s all but guaranteed that you’ll gain a better understanding of how cannabis extractions work too.