Using Quick Wash Ethanol (QWET) to Minimize Post-Processing
As far as making cannabis concentrates goes, there are several prominent methods that can be used. Ethanol extractions have a solid and growing presence among the three most popular solvent-based cannabis extraction methods today. Did you know, however, that a growing number of protocols advocate and call for cold ethanol extractions? Have you ever wondered why?
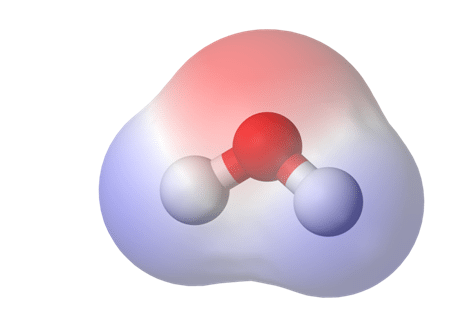
To be able to understand the reason why cold ethanol is becoming more popular among extractors, let’s review the chemical rules of solubility. Any molecule, from water to THC, can be described in terms of its polarity. When two atoms share a covalent bond but have different electronegativities (0.7<e–< 4.0), the electrons in the bonds are shared unequally, and the molecular becomes polar.
Put another way, if electrons in the molecule are distributed unevenly, a magnetic dipole is formed as one side becomes electron-rich (bearing a negative charge) and the other electron-poor (bearing a positive charge). This causes the molecule to behave like a tiny magnet. We call molecules that have this dipole polar because they have two distinct poles, one electropositive and one electronegative. Molecules where electrons are distributed evenly do not have magnetic poles and are therefore non-polar.Because of their electric charge, polar molecules are magnetically attracted to other polar molecules. Non-polar compounds do not share or take advantage of this affinity.
Figure 1. This is a heat map of electron density, a standard method of displaying bond polarity. The red region is representative of areas with high electron density, and blue represents low density1.
Solubility, then, is the process of any liquid (called the ‘solvent) engulfing and surrounding a compound (called the ‘solute’) on a molecular level if it is able to do so. How can we determine if a solute will be soluble in a solvent? One way is by looking at the relative polarity of the two compounds. A general rule-of-thumb for solubility is “like dissolves like.” That’s how we can predict that water, a highly-polar solvent, will dissolve table salt, which by definition is a polar powder. That is also how we predict that THC can be extracted by butane, supercritical CO2, and by ethanol.
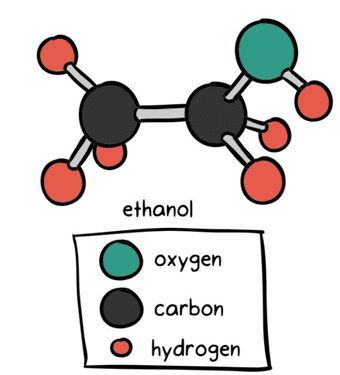
Because of its polar head, ethanol is notoriously capable of dragging water-soluble particles out of the cannabis plant during the extraction process. Ethanol not only pulls out the desirable cannabinoids and terpenes, but also extracts the cell’s chlorophyll and plant waxes.
Another facet of solubility is that hotter temperatures cause polar compounds to dissolve more readily in solution. There are two generally-accepted reasons for this. The first is a consequence of the Kinetic Molecular Theory. To dissolve, the solute molecule has to be hit by a molecule of solvent at just the right place and orientation for a molecular bond to form. Lower temperature equals less molecule movement, so less likelihood of successful molecular interactions. The second reason is that the lattice of water molecules that surround molecules inside plant cells is disrupted at higher temps. Once the lattice is broken its easier for molecules to become solvated and escape from inside.
Figure 2. ethanol contains both a polar and non-polar portion. The alcohol of a two-carbon hydrocarbon, ethanol’s ‘tail’ is made up of the electrically-neutral ethyl group, while the ‘head’ is composed of the electrically-charged oxide group2.
Cold ethanol is generally called for in the lab when the process tries to tone down its hydrophilic properties. That is because the solubility of polar, water-based molecules in ethanol is directly correlated to the temperature of the solution. Cold extractions also have the added benefit of preserving the in vivo levels of cannabinoids in whole-spectrum extracts, where the extraction of medicinal cannabinoids such as THCA or CBDA is desirable3.
The most common method for using cold ethanol to minimize extraction of water-solubles is the Quick Wash Ethanol (QWET). To maintain low temperatures, both the ethanol and the cannabis are cooled to below -20°C, ideally with dry ice. Depending on the consistency of the material and how finely it is ground, starting material is soaked for 3-10 minutes in the cold solvent. Once the cold wash is finished, routine evaporation and filtration steps follow4.
QWET has definitely been shown to reduce the earthy, musty smell of ethanoic extracts indicative of plant waxes and chlorophyll. However, the cold temperatures also somewhat decrease the efficiency of pulling out cannabinoids too. Determination of the exact parameters which maximize cannabinoid extraction while minimizing the presence of undesirables will be required in the lab. We believe QWET is a viable method for cannabis extraction which can eventually be used to eliminate the winterization step in ethanoic extracts.
References
- https://en.wikipedia.org/wiki/File:Water-elpot-transparent-3D-balls.png
- https://www.khanacademy.org/test-prep/mcat/chemical-processes/alcohols-and-phenols/v/properties-of-alcohols
- https://www.catscientific.com/dissolving-acidic-cannabinoids-for-a-thca-cbda-solution
- https://extractcrafter.com/2016/07/27/super-cooled-qwet-wash-for-cannabis-extraction-using-dry-ice