The medicinal and healing properties of Cannabis sativa are well-established from ancient times. There are hundreds of molecules, including cannabinoids, terpenes, and flavonoids, that are of interest for their medicinal merit. The utilization of cannabis-derived molecules for their therapeutic values is globally recognized and hence, the legalization of medical cannabis has been augmenting.
To use these therapeutically useful phytoconstituents in modern medicines, they must first be extracted. There are various extraction techniques used to separate the compounds native to cannabis plants and remove them from the biomass. Several common forms of cannabis extraction rely on a solvent. In brief, the cannabis plant material is soaked in solvent or a solvent system for some time frame, the plant material is subsequently removed, the liquid filtered, and the solvent removed to get a highly concentrated extract.
Distillation is a physical separation process based on differences in the boiling points of a liquid or liquid mixture. The distillation process is applied to achieve high purity, extracted phytoconstituents. There are two types of distillation: a) simple distillation and b) vacuum distillation. Simple distillations are used in distilling thermostable phytoconstituents, whereas vacuum distillations are used in the distillation of thermolabile phytoconstituents.
The miscella (oil and solvent) after the extraction of phytocannabinoids from solid-liquid extraction methods of dry cannabis biomass or hydrodynamic methods of extraction of phytocannabinoids from fresh cannabis biomass must be distilled and concentrated (solvent recovery), to get a solvent-free, highly concentrated full-spectrum extract. Also, the full-spectrum phytocannabinoids can be subjected to distillation to remove much of the tetrahydrocannabinol (THC) and produce a broad-spectrum distillate.
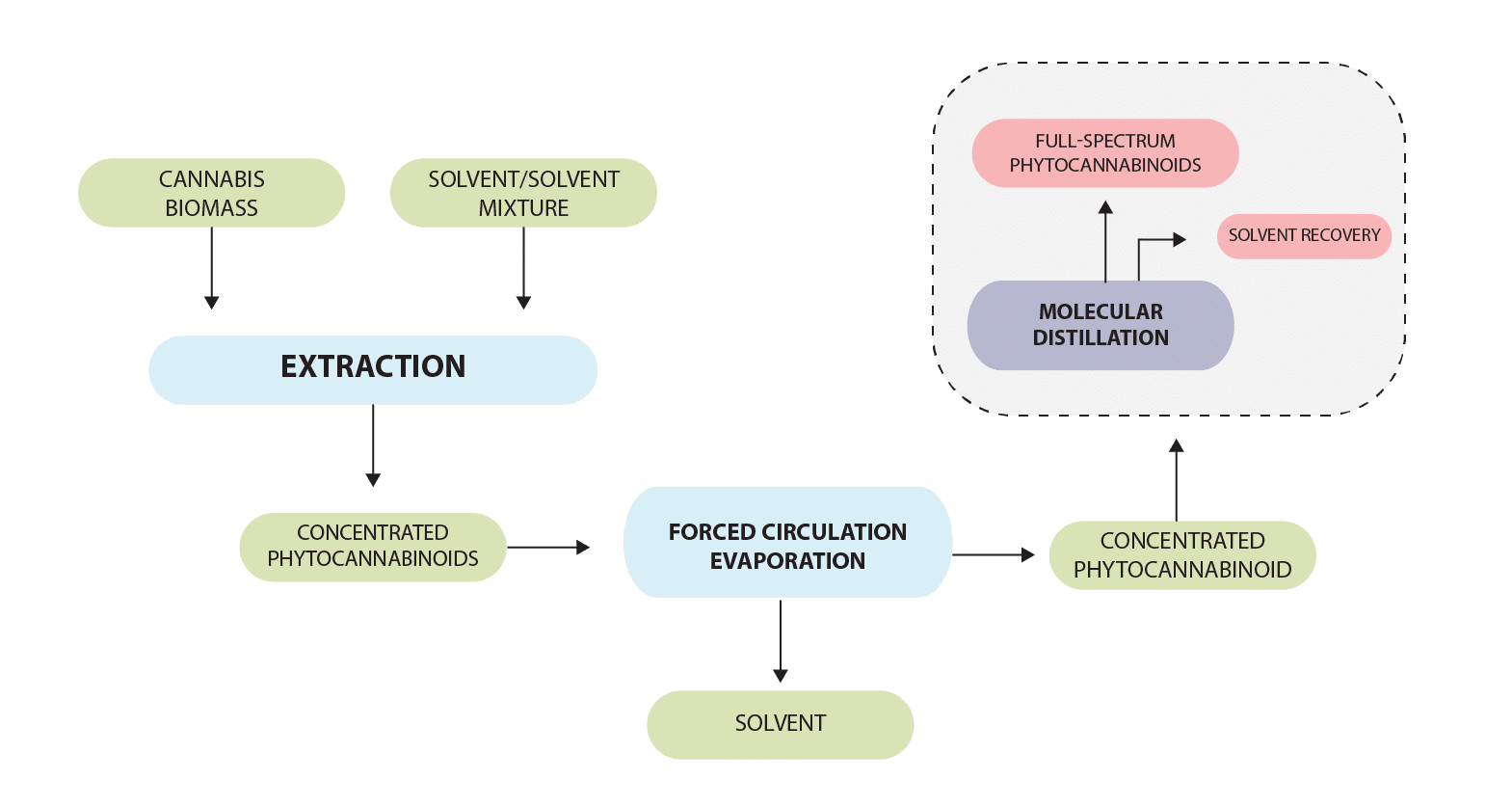
At Clean Green Biosystems, we have designed a PLC-SCADA-based method (Programmable Logic Controller-Supervisory Control and Data Acquisition) for separating molecules from cannabis, called Low Temperature Short-Contact Distillation Path, which combines forced circulation evaporation and molecular distillation. (Figure 1)
Once the extraction is complete (whether solid-liquid or liquid-liquid extraction in case of patented hydrodynamic technology [1]), the miscella (containing the extracted phytochemicals and solvent) is collected in a tank and is subsequently evaporated through a forced circulation evaporator and molecular distillation system. Depending upon the miscella volume that needs to be processed, single, double, or multistage systems may be employed.
The forced circulation evaporation system is composed of shell and tube heat exchangers, a flash evaporator, and primary and secondary condensers. The ASME-compliant shell and tube heat exchangers heat the miscella so that the vapor is created. The primary condenser distills the first-pass vapor whereas the secondary condenser further refines vapor from the primary condenser. This integration helps in efficient recovery of vapors. The forced circulation evaporation system removes around 75-80 % of the solvent from the miscella. The output of this evaporation process is concentrated miscella, which can be further purified using molecular distillation.
Components and Significance of a Molecular Distillation Unit:
Figure 1: Low temperature, short-contact distillation path designed and developed by Clean Green Biosystems.
Thermally unstable compounds can be safely purified or separated by applying molecular distillation methods. [2] Shi et al defined molecular distillation as the distillation method where the distance between the evaporator and condenser reaches to the mean free path of a vapor molecule. [3] The mean free path is the average distance traveled by a moving molecule between collisions.
Phytochemicals have characteristic vapor pressures that are related to their chemistry and the extraction solvent, a concept that’s applicable for phytocannabinoids and solvents such as ethanol, hexane, chloroform, ethyl acetate, etc. The vapor pressure of the pure solvent can be determined, but when the solvents become miscella, the entire chemistry changes, and thus the vapor pressure changes too. Because cannabis is an agricultural product, the phytochemicals vary from variety to variety, climatic conditions, agronomical practices, etc. Hence, when doing extraction, these phytochemicals alter the vapor pressures of extraction solvents. Thus, separating phytocannabinoids is greatly influenced by relative differences in vapor pressure.
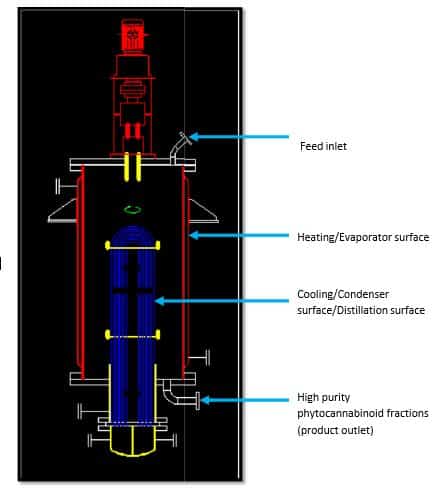
As phytocannabinoids are higher molecular weight, thermolabile compounds, the application of high vacuum distillation methods, such as molecular distillation, are used to isolate/separate/purify components without thermally degrading them. A molecular distillation unit has a feed vessel (Figure 2) that introduces miscella into the unit, whereupon the wipers spread an even coating as a thin film on the heated walls of the unit, thereby increasing the surface area. Specific and proprietary wipers can produce a thin layer of extract that’s 0.05 – 0.1 mm in thickness, significantly enhancing the evaporation surface area. The speed of the wiper blades’ rotation can be optimized and controlled to ensure uniform flow of the miscella for a rapid and efficient evaporation.
Molecular distillation uses short-path evaporators, and the distance (varies with flow and evaporation rates, but typically between 1 and 30 cm) between the evaporator and the condenser minimizes pressure drop. The distance between the heating and cooling surfaces is carefully designed to have a faster, rapid, and efficient separation of solvents from the miscella. The thermally labile molecules have short contact times with heat (may be as low as one millisecond), and thus, decomposition of the molecule(s) is kept to a minimum or does not occur at all. The evaporated solvent is condensed using chilled water, and the thick/concentrated miscella is expelled by the wipers. The solvent/solvent system is collected through an external vent condenser and can be used to extract further materials. The highly concentrated cannabis extract is collected for further processing or for direct use.
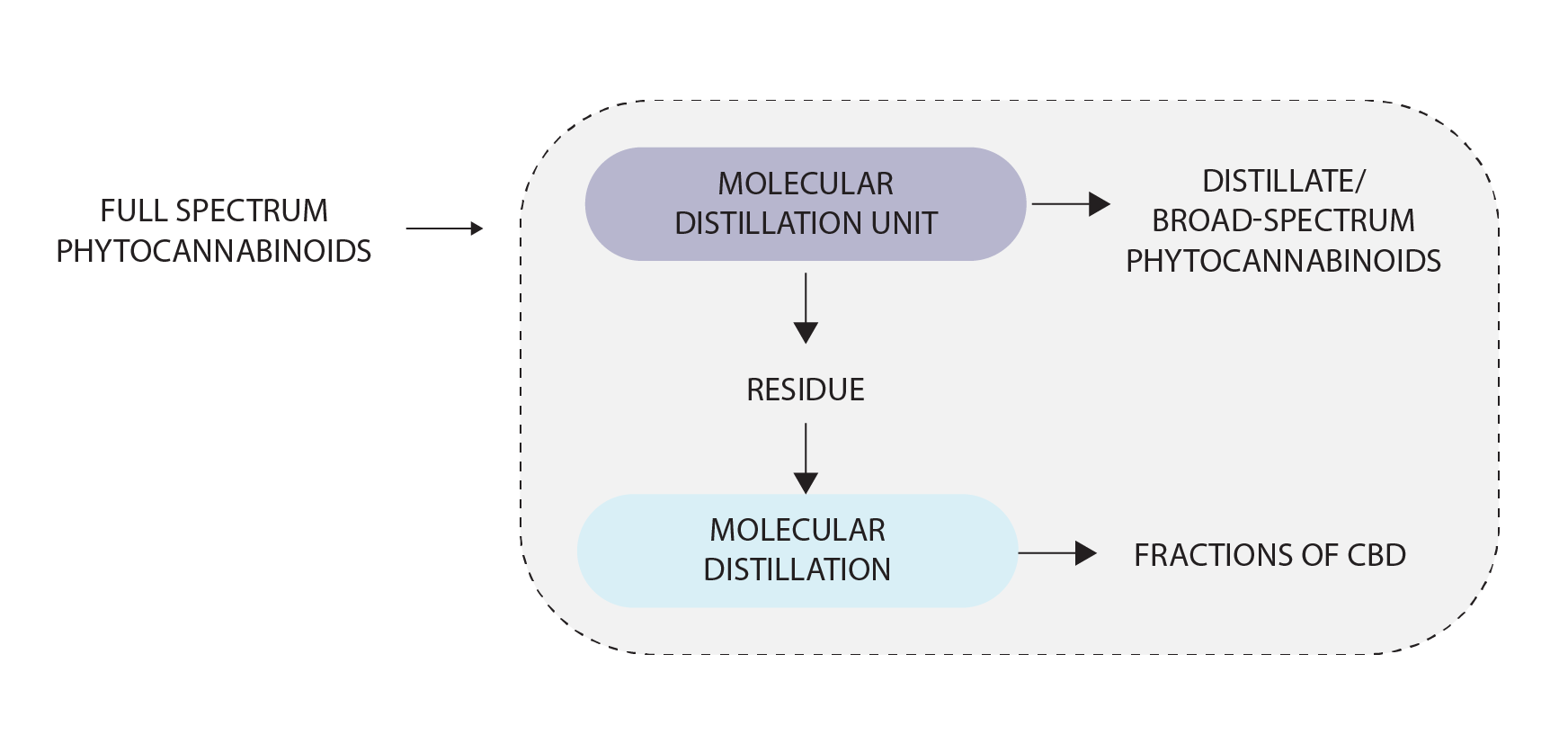
The resultant product can be further distilled to get THC-remediated, broad-spectrum product. Various fractions of the broad-spectrum phytocannabinoids can also be isolated using the molecular distillation system by adopting different standard operating procedures.
In sum, molecular distillation provides a way to retain molecular integrity and to purify thermally unstable molecules and related compounds having low volatility and elevated boiling points. The method uses low pressures (1-5 Pa, 0.01-0.05 mbar) to keep the distance between hot and condensing surfaces less than the mean free path of the molecules. All process control systems like temperature control, flow control, vacuum control, and product viscosity are controlled by PLC-SCADA software.
References:
[1] Prem S. Extraction of Full-Spectrum Phytocannabinoids Using a Hydrodynamic System. Extraction Magazine, Nov-Dec 2020.
[2] Lutisan J, Cvengros J. Mean free path of molecules on molecular distillation. The Chemical Engineering Journal and the Biochemical Engineering Journal. 1995;56(2):39-50. [journal impact factor = 13.273; times cited = 42]
[3] Shi J, Posada LR, Kakuda Y, Xue SJ. Molecular distillation of palm oil distillates. Evaporation rates, relative volatility and distribution coefficient of tocotrienols and other minor components. Separation Science and Technology. 2007;42(14):3029-3048. [journal impact factor = 1.718; times cited = 7]