Chromatography is the most widely used method to isolate pure cannabinoids for quantification [1], and it is also used to refine extracts to high purities. This technique is effective but some forms of chromatography like flash or preparative high-performance liquid chromatography may involve large amounts of expensive organic solvents and silica; flash and centrifugal partition chromatography suffer from their purification capabilities, lower chromatographic resolution and efficiencies. While supercritical fluid chromatography provides a greener, higher resolution, scalable alternative, non-chromatographic technologies capable of improving the downsides of conventional purification/isolation procedures are also being researched.
Molecularly imprinted polymers (MIPs) may satisfy the need to find a more economical and scalable method to selectively isolate cannabinoids. This affinity-based purification technique is used in chemical analysis, sensing, and material ab/adsorption. [2,3] MIPs are particularly suitable for commercial applications because of their long-term chemico-physical stability, low synthesis costs, and excellent reusability. [4,5]
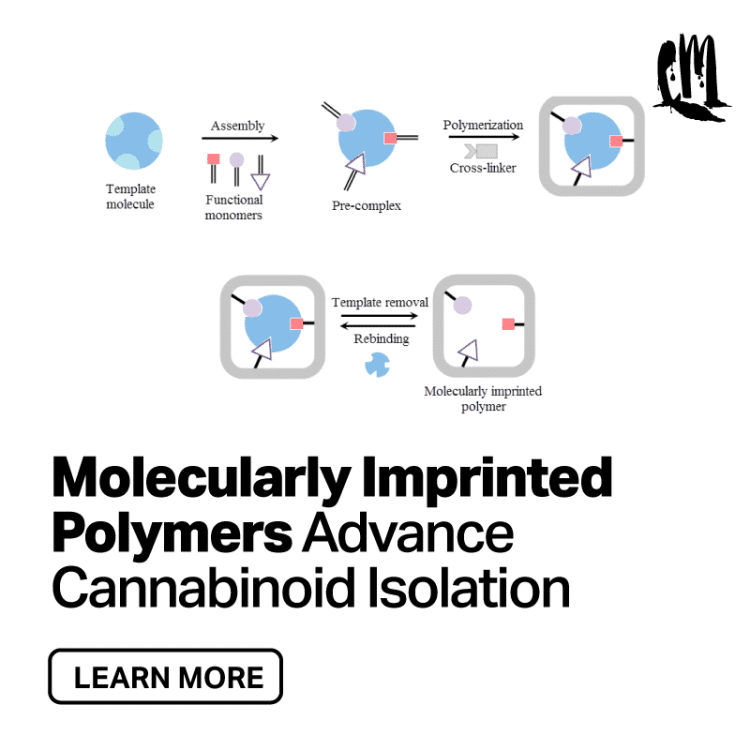
Polymers are formed by relatively small structural units called monomers, chemically linked in polymer chains or networks, and organized in various physical forms such as monoliths, microspheres, thin films, nanostructures, etc., depending on the intended application. [5]
MIPs are created with “template induced binding sites to recognize the analyte of interest.” [5] Per a recent international patent, they are macroreticular beads of monomers containing specific cavities capable of sequestering the target cannabinoid from the extract. These binding sites are obtained by co-polymerizing functional and cross-linking monomers in the presence of a cannabinoid proxy with the same molecular spatial arrangements and functional properties of the intended cannabinoid. Acting as a mold for the formation of the complexing pockets, the cannabinoid proxy can be removed from the polymerized matrix by hydrolysis and Soxhlet extraction. [6] The complementary cavity left behind is then able to specifically and selectively recognize and bind to the target cannabinoid.
Per the Affinity™ system by Sixth Wave Innovations, once absorbed, the selected cannabinoid can be separated from the mixture and recovered through ethanol wash and distillation, obtaining a highly concentrated and pure cannabinoid.
Each cannabinoid has a different chemical structure and can interact differently with the surrounding chemical groups in the binding cavity of MIPs; exploiting these chemical differences, cannabinoid discrimination is said to be possible during the purification process, creating, for example, tetrahydrocannabinol (THC)-free extracts or specific formulations containing only the desired compounds. This, of course, should be verified and validated with analytical data.
It has been shown that ultrasound technology is capable of increasing polymerization yields and speeding up polymerization processes. [7] Ultrasonic probes can improve MIP quality, controlling the morphology of polymers. [7] Hielscher notes that ultrasound also increases the rate of proxy cannabinoid desorption to obtain active MIPs by promoting the solubility, diffusivity, and movement of solvent and template molecules.
It remains to be seen if MIP technology will replace or supplement other traditional cannabinoid purification and isolation methods.
References:
[1] Lazarjani MP, et al. Methods for quantification of cannabinoids: a narrative review. Journal of Cannabis Research. 2020;2(35). doi:10.1186/s42238-020-00040-2. [Times cited= n/a] [Journal impact factor= n/a]
[2] Gao M, et al. Recent advances and future trends in the detection of contaminants by molecularly imprinted polymers in food samples. Frontiers in Chemistry. 2020;8. https://doi.org/10.3389/fchem.2020.616326 [Times cited= 1 (Semantic Scholar)] [Journal impact factor= 3.994]
[3] Lendoiro E, et al. Molecularly imprinted polymer for selective determination of Δ9-tetrahydrocannabinol and 11-nor-Δ9-tetrahydrocannabinol carboxylic acid using LC-MS/MS in urine and oral fluid. Analytical and Bioanalytical Chemistry. 2014;406:3589-97. doi:10.1007/s00216-013-7599-1 [Times cited= 21 (Semantic Scholar)] [Journal impact factor= 3.637]
[4] Lowdons JW, et al. MIPs for commercial application in low-cost sensors and assays – An overview of the current status quo. Sens Actuators B Chemical. 2020;325(128973). https://doi.org/10.1016/j.snb.2020.128973 [Times cited= 5 (Semantic Scholar)] [Journal impact factor= 7.1]
[5] Paradeshi S, Singh SK. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. Royal Society of Chemistry. 2016;28. doi:10.1039/c6ra02784a [Times cited= 52] [Journal impact factor= 3.119]
[6] Sanchez-Gonzalez J, et al. Cannabinoids assessment in plasma and urine by high performance liquid chromatography-tandem mass spectrometry after molecularly imprinted polymer microsolid-phase extraction. Analytical and Bioanalytical Chemistry. 2017;409(5):1207-1220. DOI 10.1007/s00216-016-0046-3 [Times cited= 25 (Semantic Scholar)] [Journal impact factor= 3.286]
[7] Viveiros R, et al. Green strategies for molecularly imprinted polymer development. Polymers. 2018;10(3). https://doi.org/10.3390/polym10030306 [Times cited= 21 (Semantic Scholar)] [Journal impact factor= 3.426]
Image: Saylan Y, Akgönüllü S, Yavuz H, Ünal S, Denizli A. Molecularly imprinted polymer based sensors for medical applications. Sensors. 2019; 19(6):1279. https://doi.org/10.3390/s19061279. CC BY-4.0