There is no doubt that excess carbon dioxide (CO2) hurts our planet. The trendlines direly speak of rising sea levels, vanishing sea ice, and diminished marine populations juxtaposed to more heatwaves, wildfires, and surging taxation of an energy grid to keep us cool in hotter and hotter geographies. Atmospheric CO2 amplified a lot with the advent of industry and modern concepts like traffic.
And yet, it is CO2 and other natural greenhouse gases that have permitted a habitable and hospitable Earth by warming its surface beyond what would otherwise have occurred. Like most things, is a balance and a tipping point, and a point of no return. While CO2 may be a regular villain, the good news is it can be harnessed and put to work for the greater good of our planet. The surplus of CO2 waste makes it very cheap, and serendipitously, applications using CO2 as an extraction solvent have existed for a long time. For example, Ernst B. Auerbach demonstrated in 1931 that constituents in botanical extracts could be made soluble in liquid CO2. [1]
These days, this is all very well-known as sub-, and supercritical CO2 (scCO2) has been utilized for cannabis extraction and vast fields of many other plants canvassing the earth. [2] Auerbach mentioned that his invention provided “a process by means of which oils may be treated and purified without the use of distillation or chemical decomposition” and that separated “oils substantially in the order of the molecular weights of constituent parts thereof,” the latter highlighting the lure of CO2 for its tunability.
One of the main aspects to address when considering scCO2 for extraction regards the polarities of the molecules desired. Will scCO2 only pull out the non-polar ingredients, given its non-polar similarity to hexane? The “shape” of the CO2 molecule is linear (Figure 1), and the dipole moments cancel out, as both insatiable oxygens try to tug electrons away from poor carbon, resulting in non-polarity. This is why scCO2 has often been evaluated and implemented to supplant the use of hydrocarbon solvents. After all, the old adage says, “like dissolves like.”
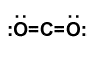
Interestingly, though, one study showed that multiple molecules containing hydrocarbon chain moieties were not very soluble in CO2. [3] The study considered 130 surfactants, polymers, and other molecules. Only about 25 of the studied molecules showed miscibility or near miscibility at specific CO2 pressures. What is more, some studies have discussed the polar nature of CO2, which may come as quite a shocker. [4]
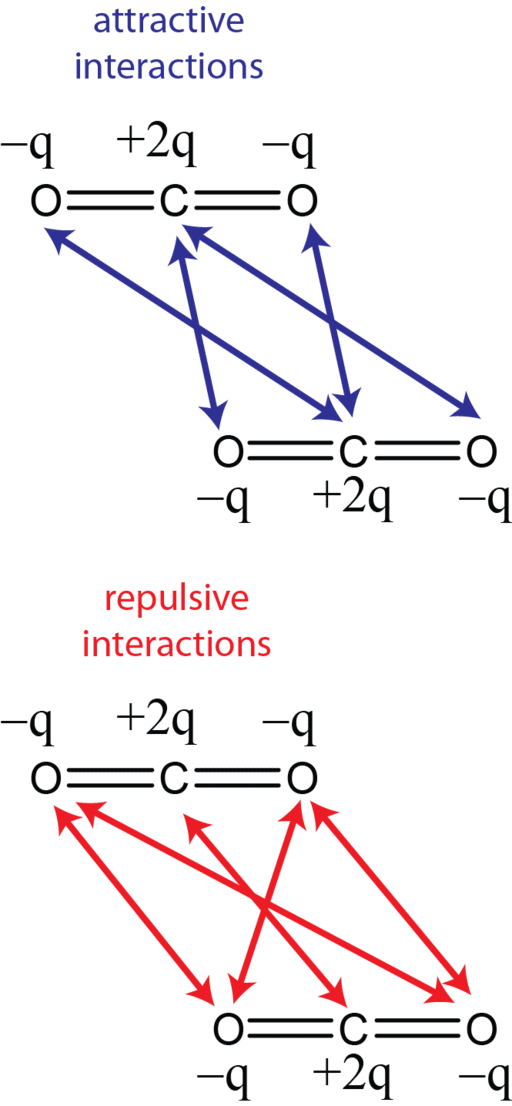
While CO2 may not have a dipole, it does have a quadrupole (Figure 2) and has been likened in solvating power to dioxane. CO2 has also been labeled as a weak Lewis acid and a weak Lewis base. I know, I know. It may have been a long time since you have met these terms. Briefly, a Lewis acid accepts an electron pair from a “donor,” aka the Lewis base, which has spare electrons not involved in chemical bonds (see the lone pair electrons in Figure 1).
The electron-deficient carbon atom in CO2 plays the Lewis acid. The more electronegative oxygens (with their lone pairs) can be Lewis bases involved in hydrogen bonding with Lewis acids. Viewing CO2 in this Lewis acid/base light is suggestive that it can solubilize polar and non-polar molecules through site-specific solute/solvent interactions. [4] An example of CO2’s polar nature can be found in its higher solubility in water than in carbon monoxide.
Dipole-quadrupole interactions with solutes are postulated as the cause of CO2’s polar nature due to the charge separation from interactions between partial negative charges on the oxygens and the partial positive charge on carbon. [4] In this regard, CO2 resembles water, the most polar solvent of all! This chemistry creates site-specific interactions. The authors report that “one should regard CO2 as a polar molecule with two active and considerably strong bond dipoles.” Although these dipoles cancel each other out, “the zero-sum is not relevant to understanding CO2 solvation from the microscopic point of view.” What is more, CO2 molecules also resemble water molecules through their ability to self-assemble. Water molecules interact with each other via hydrogen bonds that consequently affect their solvating power, and individual CO2 molecules can aggregate into dimers and trimers, which would have a corresponding effect on solvency. [4]
scCO2 is often not considered for common polar molecules like carbohydrates, but one study showed that acetylating (adding a CH3CO group) the polysaccharides made them much more “CO2-philic,” demonstrating the polar characteristics of CO2. [5]
The range of applications for which CO2 can be useful as a solvent is changing. As researchers and industries attempt to replace noxious solvents with more environmentally friendly options, it is becoming increasingly important not to write off CO2 as only having utility for capturing non-polar solutes.
References
- Auerbach E. Process for treating, separating and purifying oils. Patent US1805751A.
- Lupoi J. From gaseous monster to superhero for green extraction and purification. Extraction Magazine. Jan-Feb 2021.
- Consani K, Smith R. Observations on the solubility of surfactants and related molecules in carbon dioxide at 50 °C. J. Supercrit. Fluids. 1990;3:51-65. [journal impact factor = 3.744; times cited = 221 (Semantic Scholar)]
- Raveendran P, Ikushima Y, Wallen SL. Polar attributes of supercritical carbon dioxide. Acc Chem Res. 2005;38(6):478-485. [journal impact factor = 20.832; times cited = 194 (Semantic Scholar)]
- Raveendran P, Wallen SL. Sugar acetates as novel, renewable CO(2)-philes. J Am Chem Soc. 2002;124(25):7274-7275. [journal impact factor = 14.612; times cited = 128 (Semantic Scholar)]
Image Credit: Bernhard Kuemel <[email protected]>derivative work: Regi51, CC BY-SA 2.0; Christopher Rowley, CC BY-SA 4.0