Mushrooms of all sorts dazzle humans (and likely other animals) with their mysteriousness, magical medicinal properties, and their potential for danger which must be respected. The industry is growing like never before at a pace Mckenna could never imagine. What started as a single shop is now a whole nationwide chain, like in the case of Funguyz medical mushroom dispensary and many others.
Lore is laden with mushrooms that enable transcension from the daily minutiae known as reality into another world where our senses and neurons and innate knowledge come alive. Go ask Alice. I think she’ll know about these things.
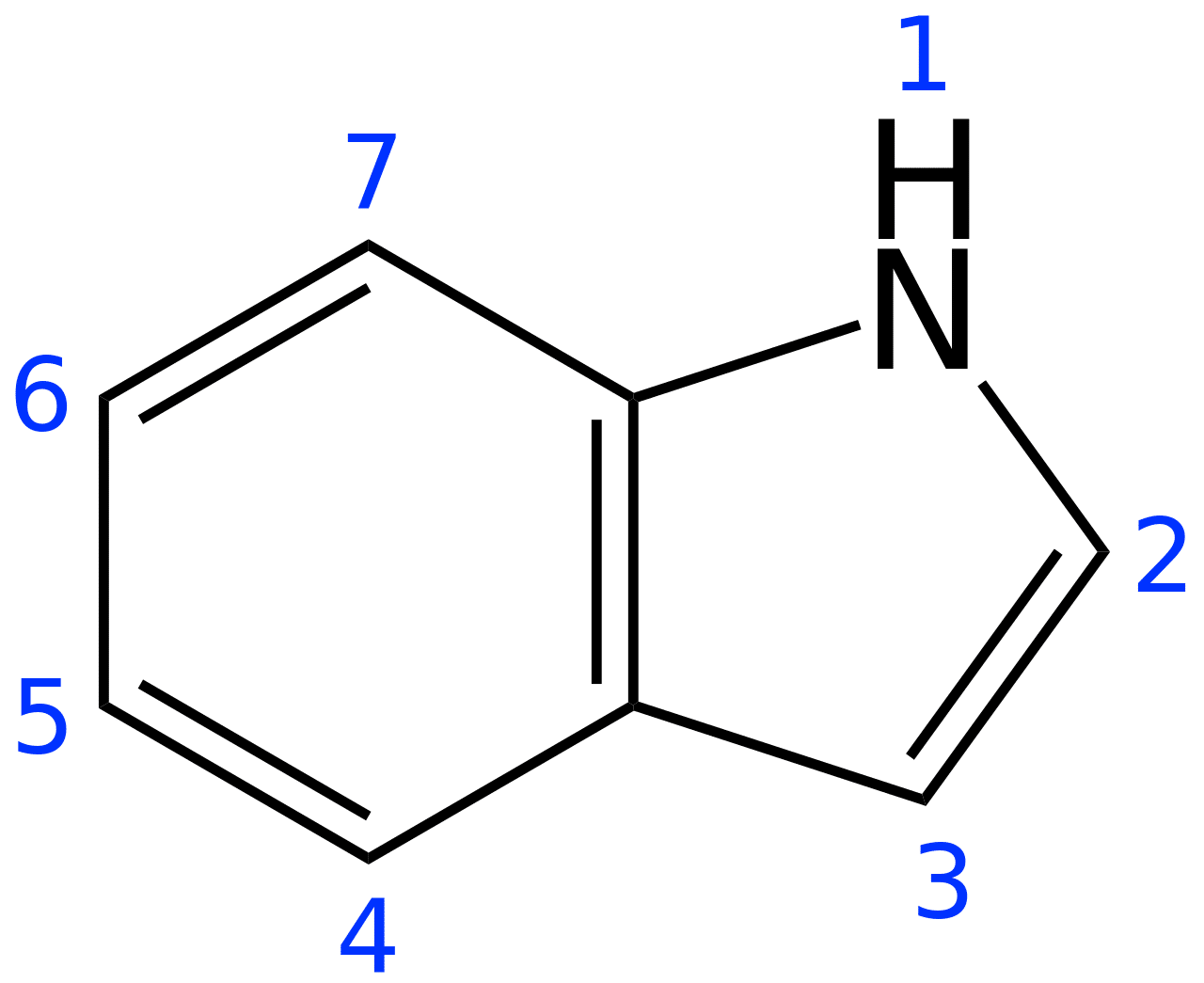
There are 600+ alkaloids, including molecules like N,N-dimethyltryptamine (DMT), ibogaine, and psilocybin, that have an indole group [1] — a beautiful bicyclic structure that marries a six-membered benzene ring with a five-membered pyrrole ring (Figure 1). A pyrrole ring contains 4 carbons, 5 hydrogens, and 1 nitrogen (C4H4NH). These alkaloids are further classified as derivatives of lysergic acid (ergoline alkaloids), tryptamines (DMT, psilocybin), carbolines, and iboga alkaloids (ibogaine). [1]
Figure 1: Structure of an indole
A paper straight out of the 1980s highlighted a method for psilocin and psilocybin extraction from Psilocybe cubensis. [2] Other extraction methods were known such as using methanol which co-extracts other useful molecules like urea, plant steroids (like ergosterol), sugars, and analogs of psilocybin (like baeocystin). [3,4]
Using methanol isn’t the best option for extraction (toxicity and selectivity), and the polarity of psilocin and psilocybin meant an aqueous, polar solution might be advantageous.
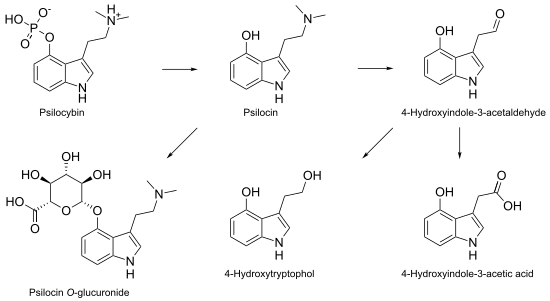
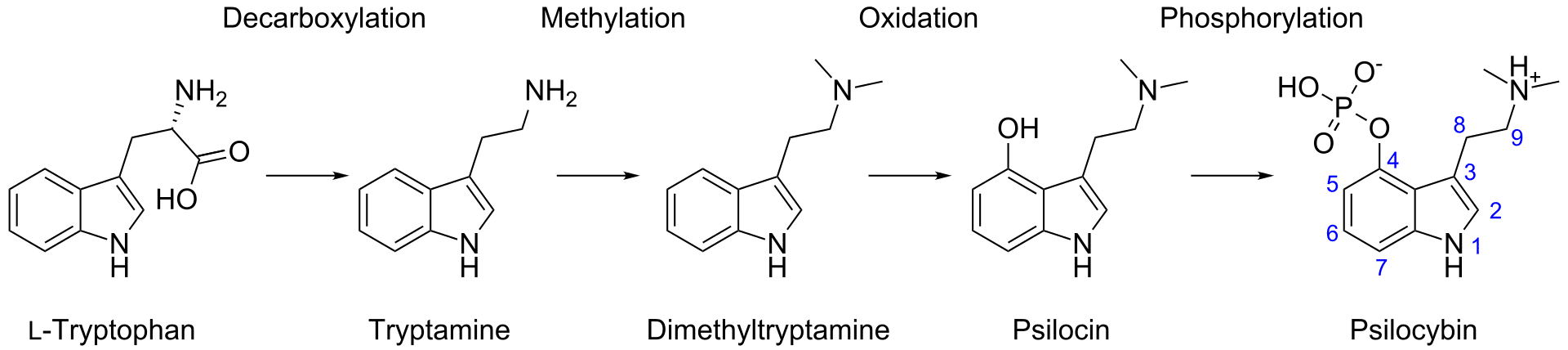
In our bodies, psilocybin gets dephosphorylated (removal of phosphate group) to psilocin (Figure 2), and it’s psilocin that conveys much of the trademark activity. Interestingly, this reaction occurs in reverse within the mushroom, as the amino acid tryptophan goes through a transformative dance to tryptamine, 4-hydroxy-DMT, psilocin, and finally psilocybin. [5]
It’s been hypothesized that N,N-DMT is created en route to psilocybin [6] (Figure 3), but researchers like Ann and Alexander Shulgin questioned: why had DMT never been measured in mushrooms? [7]
The extraction and yield of psilocybin depend on six main factors [2,8]:
- Glucose concentration – no glucose means no psilocybin production
- Ammonium succinate (a source of nitrogen) – low levels mean low yields
- Growing medium should be below pH 7
- Time – maximum psilocybin levels occur seven days following germination
- Temperature – psilocybin and psilocin perish in harvested mushrooms left in ambient conditions
- Oxidation – psilocin oxidizes, producing a bluish hue
Figure 2: Reaction mechanism for dephosphorylation of psilocybin to psilocin (and other reactions within our bodies).
Figure 3: One suspected biosynthetic pathway of psilocybin
The extraction protocol [2] used 2 to 10 grams of pulverized mushrooms, the powder mixed with 100 mL of dilute acetic acid in a beaker and titrated to pH 4 with concentrated (glacial) acetic acid. After this mixture incubated for 1 hour, it was put in a boiling water bath for 8 to 10 minutes or until the solution tested at 70°C.
This heating step dephosphorylated psilocybin to psilocin. The acid mixture was separated from the raffinate, brought to pH 8 with ammonium hydroxide, and extracted with two 50-mL aliquots of diethyl ether. The OChem foxtrot continued as the ether was dried over sodium sulfate, producing a greenish, psilocin precipitate that could be crystallized to a white powder using a 1:3 ratio of chloroform to heptane.
The benefit of this method resides in the product purity. Psilocin and psilocybin can be preferentially plucked from the mushrooms due to their solubility in the aqueous acetic acid, but many of the other molecules are left behind. [2]
This study serves as an example of how things were once done. It offers a method for psychedelic mushroom extraction. As these resurging psychedelic substances continue their ancient genuflection before humanity on forest or jungle floors, or perhaps just popping up in piles of dung, a brave new world of botanical extraction emerges just as we’ve witnessed with cannabis.
References
- Schultes R. Indole alkaloids in plant hallucinogens. Journal of Psychedelic Drugs. 1976;8(1):7-25. [journal impact factor = 1.859; times cited = 10 (Semantic Scholar)]
- Casale JF. An aqueous-organic extraction method for the isolation and identification of psilocin from hallucinogenic mushrooms. J Forensic Sci. 1985;30(1):247-250. [journal impact factor = 1.436; times cited = 13 (Semantic Scholar)]
- Beug MW, Bigwood J. Quantitative analysis of psilocybin and psilocin in Psilocybe baeocystis (Singer and Smith) by high-performance liquid chromatography and by thin-layer chromatography. J Chromatogr. 1981;207(3):379-385. [journal impact factor = 4.049; times cited = 42 (Semantic Scholar)]
- Koike Y, Wada K, Kusano G. et al. Isolation of psilocybin from Psilocybe argentipes and its determination in specimens of some mushrooms. Journal of Natural Products. 1981;44:362-365. [journal impact factor = 4.257; times cited = 32 (Semantic Scholar)]
- Fricke J, Blei F, Hoffmeister D. Enzymatic synthesis of psilocybin. Angew Chem Int Ed Engl. 2017;56(40):12352-12355. [journal impact factor = 12.959; times cited = 35 (Semantic Scholar)]
- Wieczorek P, Witkowska D, Jasicka-Misiak I, et al. Chapter 5 – Bioactive alkaloids of hallucinogenic mushrooms. Studies in Natural Product Chemistry. 2015;46:133-168. [journal impact factor = 6.92; times cited = 5 (Semantic Scholar)]
- Shulgin A, Shulgin A. TIHKAL: The Continuation. Transform Press Berkeley; 1997.
- Catalfomo P, Tyler V. The production of psilocybin in submerged culture by Psilocybe cubensis. Lloydia. 1964;27(1):53-63. [journal impact factor = 4.257; times cited = 24 (Semantic Scholar)]
Image credits: Sergei Tokmakov, Esq. from Pixabay; Jynto, CC0, via Wikimedia Commons; Ed (Edgar181) – Public domain, via Wikimedia Commons; Ed (Edgar181), Public domain, via Wikimedia Commons