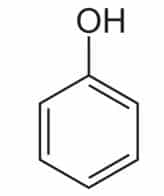
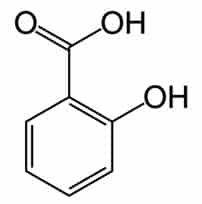
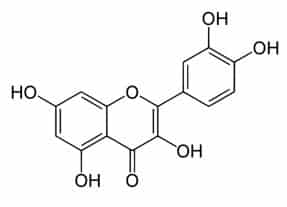
Plants produce phenolic chemicals (aka phenols or phenolics) as secondary metabolites for defense and preservation, although they also play roles in metabolism. [1] A number of these phytochemicals are valued by humans for antioxidant, anti-inflammatory, and other medicinal properties. Chemically, they consist of an aromatic ring with a hydroxyl group (OH); the most basic is phenol with a chemical structure of C6H5OH (see figure 1). Phenolic acids like as salicylic acid, C₇H₆O₃ (figure 2) contain phenol and carboxylic acid groups. Polyphenols are more complex, having multiple phenol units or subunits. This category includes flavonoids such as quercetin, C15H10O7 (figure 3). [1]
Figure 1: Phenol. Public domain.
Figure 2: Salicylic acid. Public domain.
Figure 3: Quercetin. Public domain.
It’s been estimated that there are 50,000 distinct polyphenols in nature. [2] Due to the powerful potential of these chemicals for dietary and therapeutic applications, Stalikas [1] presented an “extensive overview of methods” in 2007 that covers the extraction, separation, and detection of various phenolic acids and flavonoids. The report summarizes several decades of previous studies (up to 2007) and offers a guide to the most useful techniques incorporated therein.
Sample preparation, to start, usually required two or more steps. One was the pre-digestion step. Enzymes—α-amylase, for example—were combined with hydrolysis to promote the release of phenolic acids. This type of hydrolysis, in addition to acidic and basic hydrolysis, was commonly used for the same purpose on flavonoids. Due to complex matrices (e.g., plant material) and the sheer variety of compounds, the properties of the target compound(s) proved the only true guide for sample preparation.
Continuing to extraction, organic solvents (e.g., ethanol) could be diluted with water to reach the most polar phenolic acids. Alternatively, when using supercritical carbon dioxide (CO2), organic solvent could be added to boost the yield of polar compounds. Microwave-assisted extraction was reported to improve efficiency significantly. Solid-phase extraction with C18 silica as the sorbent and “slightly acidified” solvents and solution was perhaps the most prevalent option for the isolation step. Chromatography methods, such as counter-current chromatography, were also performed for certain compounds, such as catechin gallates (flavonoid) from tea.
For analysis of the phenols in the extract, the author points to high performance liquid chromatography (HPLC) as the technique that “dominated the separation and characterization of phenolic compounds.” HPLC column parameters varied but are outlined in the report as follows:
- Reverse-phase C18
- Length: 100 to 250 mm
- Internal diameter: 3.9 to 4.6 mm
- Particle size: 3 to 10 μm
Ionization could be avoided by maintaining a pH between 2 to 4 in the mobile phase (acids/buffers used for this purpose). For example, methanol, water, and acetic acid were used to separate phenolic acids such as caffeic acid. Gradient elution by polarity (with less polar phenols retaining for longer) proved a viable strategy when multiple analytes required separation.
Ultraviolent/visible (UV/Vis) detectors were the primary identification tool since the aromatic rings of polyphenols absorb light in this region of the electromagnetic spectrum (~200–370 nm; 280 nm for combined separation was typical). Additionally, a photodiode array detector was used since it “allows for scanning real time UV/Vis spectra of all solutes passing through the detector.” Mass spectrometry (MS) and/or nuclear magnetic resonance (NMR) in tandem with chromatography provided more powerful results.
Ultra-performance liquid chromatography (UPLC), with greater pressures and smaller particle sizes, could be coupled with MS to successfully process higher throughput.
Ultimately, the report concludes that “[t]he overall analytical method to be used for these compounds is not cut-and-dried but is highly dependent upon the matrix characteristics, the availability of the techniques, the selectivity, and the interest in structure elucidation and unambiguous identification.” [1]
References
- Stalikas C. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30:3268-3295. doi:10.1002/jssc.200700261. Impact Factor: 2.516; Times Cited: 586 (ResearchGate)
- Ziaullah HPVR. Chapter 1: Application of NMR spectroscopy in plant polyphenols associated with human health. In: ur-Rahman A, Choudhary MI, eds. Applications of NMR Spectroscopy. Bentham Science Publishers; 2015:3-92. doi:https://doi.org/10.1016/B978-1-60805-999-7.50001-X
Image: Nabin Mewahang from Pixabay