Every extraction strategy has its strengths and shortcomings. We often think about costs, yields, throughputs, regulatory controls, etc., but an initial aspect of one’s extraction blueprint must be the consideration of solvent polarity. When we say a molecule is polar, that molecule is amped up with electrical charges, positive and negative. The polarity stems from the individual atoms that comprise the molecules. Some atoms are electron hogs; others are electron philanthropists, like it or not.
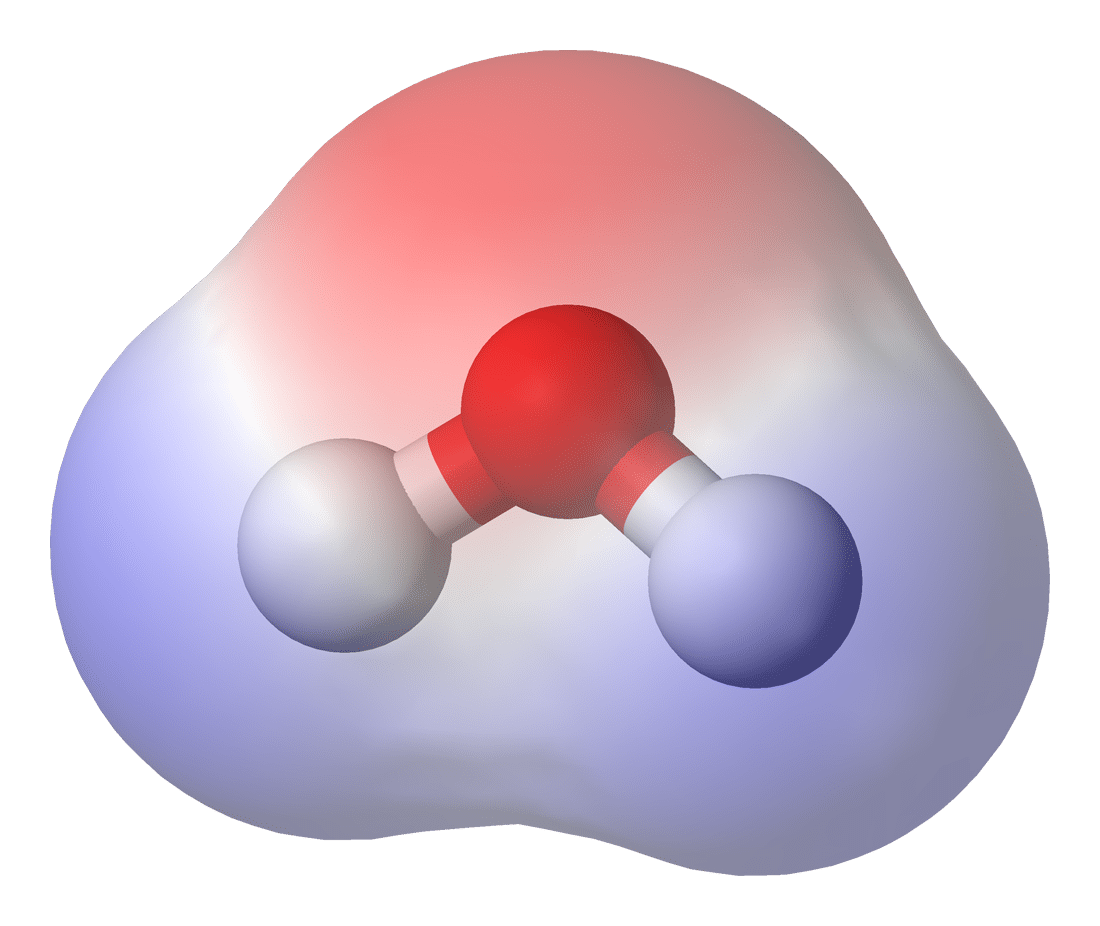
Consider water with its two hydrogens and one oxygen. The oxygen atom has a higher electronegativity (O = 3.5; H = 2.1), meaning it pulls electrons towards it in a molecular tug-of-war with the hydrogen atoms. Because the negatively charged electrons are pulled closer to oxygen, this creates a localized region of negative charge, whereas the region that used to contain those electrons becomes slightly positively charged. (See Figure 1 where red = negative and blue = positive.)
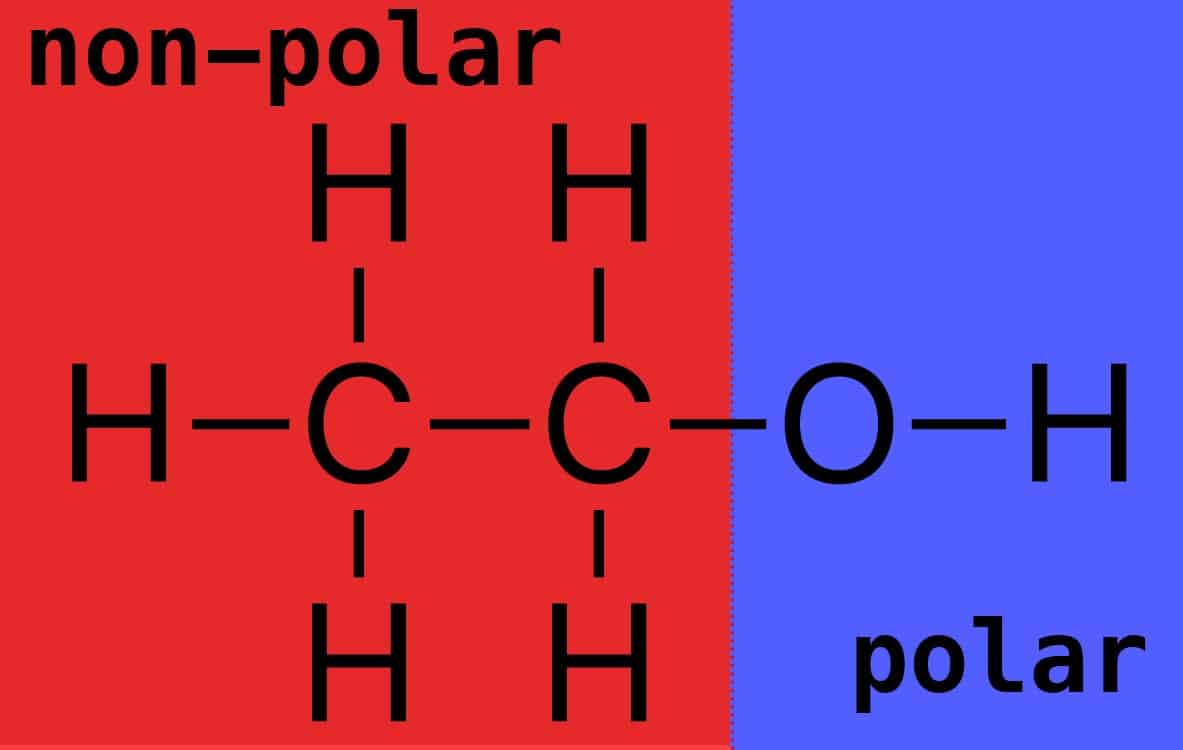
Ethanol is polar, but not as polar as water. Rather, those carbon atoms and extra hydrogens in C2H5OH bring more non-polar power to face off against the electron bully known as oxygen. (Figure 2)
The carbon and hydrogen atoms have relatively similar electronegativities (2.5 and 2.1, respectively), creating the non-polar region, whereas oxygen pulls electrons towards it from its adjacent carbon and hydrogen partners. So, there’s a non-polar and a polar region.
Carbon dioxide (CO2) is non-polar since it’s a linear molecule (O=C=O), and therefore the slight negative charges on each end cancel each other out. Supercritical CO2 is also non-polar; however, it can be used to dissolve slightly polar, low-molecular weight species. Increasing the pressure can augment the solubility of more polar species, but this may not be a practical decision.
So, why is all of this negativity important? The polarity of the solvent, in turn, identifies which types of molecules it can dissolve, since “like dissolves like.” Therefore, sCO2 and water can preferentially extract very different phytochemicals. And then there’s subcritical water.
Subcritical water has different properties than regular, liquid water that decrease its polarity, transforming its solubilizing capacity to extract molecules over different polarities thereby resembling an organic solvent like ethanol.
These properties can be conjoined via the use of co-solvents. Often, researchers using sCO2 will evaluate their extraction strategies and yields using sCO2 versus sCO2 and ethanol or some other polar solvent. The co-solvent might make up just a few percent of the overall solvent or it may be much higher.
For cannabis extraction, obviously the three common methods include hydrocarbon, sCO2, and ethanol. Of these, ethanol is the most polar. Some extractors prefer ethanol because of its mid-range polarity when considering polarity indices, which means non-polar and polar molecules can be extracted. Cannabinoids and terpenes, however, are not polar molecules, which is why hydrocarbons and sCO2 work well.
For other botanicals like mushrooms, the extractor needs to research which molecules are of interest and which will dissolve in a chosen solvent. There may be polysaccharides and triterpenoids that are water-soluble, or terpenes that are not. The trick is to scope out which solvents will be suitable by understanding the chemistry involved and fleshing out what will or won’t work.