Sometimes, unbeknownst to us, weird things happen. Perhaps it’s just one thing or a series of seemingly unrelated things. These things might occur silently, or with a sudden bang. Sometimes, the subsequent events are problematic; others are opportunistic.
Case in point: beer.
It’s thought that ancient Sumerian farmers accidentally fermented some of their grain through moisture and heat and, of course, the yeasts needed to ferment plant sugars into ethanol. Being tasty stuff, the invention of beer encouraged advanced agricultural techniques. And drinking the beer may have led to a longer life, since water supplies were often fouled via animal waste. The safety of beer over water has been demonstrated several times historically, including during the Great Bubonic Plague. Saint Arnold knew this, saving his Belgian community by convincing them to drink beer.
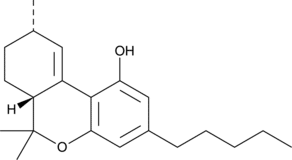
There are new and accidental discoveries in the cannabis industry as well. This is the story of delta 10-tetrahydrocannabinol (Δ10-THC) and its mysterious formation and crystallization following distillation.
A California cannabis company, Fusion Farms, purchased some outdoor-grown cannabis to get their extraction business off the ground. At the time, wildfires raged in California, scorching the earth, communities, and small plots of cannabis plants. Aerial firefighters dropped fire retardants, which winds helped migrate away from intended targets and onto outdoor crops like cannabis. Although unaware at the time, Fusion Farms had purchased cannabis biomass contaminated with fire retardant.
Back at Fusion Farms’ processing facility, after extracting the cannabis biomass and distilling the extract to remove any non-volatile fire retardant components, they noticed unusual crystals forming in the distillate. What was this? The crystals couldn’t have been THCA, which isn’t present after high-temperature distillation, and they didn’t look like any other cannabinoid crystal structure the team had encountered. So, through repeated recrystallization, the team purified the solid and began instrumental analysis to identify the unknown crystals. Fusion Farms used their internal HPLC and found a peak that closely aligned with cannabichromene (CBC), but wasn’t an exact match. Then, with the help of a licensed testing facility, they obtained further HPLC data to compare with all other known cannabinoid reference standards. But no match was found, and for several months no conclusion could be reached.
I spoke with Josh Jones, Ph.D., organic chemist and founder of Jonesing Labs LLC., who consults for Fusion Farms. “The first thing I wanted to see was nuclear magnetic resonance [NMR] for the unknown crystal,” he began. NMR spectroscopy is a powerful analytical method for determining chemical structures. “If you have a purified substance, NMR allows you to ‘see’ the structure of a molecule. It’s a wonderful tool,” says Josh. NMR instruments can be tuned to see only certain atoms, and to determine where those atoms are in relation to other atoms in the molecule. This provides more information than HPLC, GC or mass spectrometry, but NMR isn’t typically available at cannabis testing facilities.
So, a chunk of the isolated crystals was taken to a specialty lab for NMR analysis. The spectral data indicated Δ10-THC.
“Prior to this NMR data, a lot of people had been seeing this mystery compound show up as a minor component on their distillate COAs, but they thought it was CBC,” Josh said. “Without having a certified reference standard for Δ10-THC, typical HPLC analysis can misidentify Δ10-THC as CBC or CBL (cannabicyclol.) We looked a bit deeper at the HPLC data, especially using 2D HPLC scans, which show absorbance at multiple UV wavelengths. We then saw a difference between the isolated Δ10-THC and standards for CBC and CBL. In combination, the 2D HPLC and NMR data make a strong case for identifying Δ10-THC as the unknown crystals formed in Fusion Farms’ distillate. Then, as luck would have it, a certified Δ10-THC reference standard was released, which agreed with the GC and mass spectrometry data we collected.”
Fusion Farms was convinced that fire retardant contamination had something to do with the production of Δ10-THC. What else could it have been? Subsequent attempts to reproduce the Δ10 conversion using different batches of biomass were unsuccessful, which likely meant something in the outdoor grown batch was responsible. Fusion Farms, thinking that perhaps they’d stumbled onto something useful for further scientific inquiry and market potential, wanted to produce larger volumes of Δ10-THC, but in a controlled and reproducible way. So, they asked Josh to reverse engineer the Δ10-THC reaction mechanism and to design a method for production. Although Raphael Mechoulam published a paper in 1984 that described the synthesis of Δ10-THC [1], the method used harsh chemicals and isn’t suitable for production of cannabis-derived products. His clients were hoping for a “greener” method, so Josh set out to recreate the process in a different way.
After screening various conditions, Josh found the combination of several food-grade additives can act as a catalyst to bring about the conversion of Δ9-THC to Δ10-THC, but not in high yields. To improve yields, he considered what reaction mechanisms might favor the conversion and postulated that radical formation could facilitate “migration of the Δ9 double bond to the Δ10 position.” However, radicals (or ‘free radicals’ in biology) are highly reactive unless stabilized by the right conditions. (In physiology, for example, uncontrolled free radicals often cause cellular damage and disease.)
To test this radical hypothesis, Josh found that including catalytic amounts of a food-grade radical initiator, along with some other components to stabilize the reactivity of radicals, provided a 75-80% yield of Δ10-THC. “[This radical initiator] is an interesting thing. There’s a long history of its use to modify the structure of terpenes, and this actually allowed early organic chemists to discover the structures of various terpenes. Since cannabinoids are [biosynthetically] derived from terpenoids, I thought it might be a suitable additive for this reaction. But intentionally introducing radicals is a delicate thing, where you need to tailor the conditions to get the result you want. Too much of a radical initiator can decompose the molecules. Using just a tiny amount, along with some other food-grade components to subdue radical activity, allows this reaction to work,” Josh explained. It’s been an exciting project that Fusion Farms and Jonesing Labs have taken on together, and their process is currently patent pending.
The burning question yet to be discussed is what does Δ10-THC actually do, from a physiological and medicinal vantage point? While investigations into these questions are scant, Mechoulam’s lab found that pigeons got less stoned with Δ10-THC than after Δ9-THC ingestion. [2] Fusion Farms is in the process of determining any specific beneficial effects of Δ10-THC, so stayed tuned.
The Holy Grail for cannabis researchers is to discover and understand new uses for the plant and its unique secondary metabolites. Fusion Farms, Josh, and his team have applied some really cool science towards understanding and utilizing the chemistry of Cannabis sativa. This opportunity to explore new chemistries of cannabinoids shows how the scientific method can guide progress through applying disciplines from more traditional industries. These efforts add another shade of color to the scene regarding why many scientists like Josh and me cherish our career and relationship with cannabis.
These findings resulted from the chance observation of something unusual (as it is with many discoveries and inventions), and the persistence to learn about how it happened. Happenstance chemical interactions, things that may not otherwise be pursued, can lead to discovery and inventions of new methods for producing something altogether different. Only science and time will enable Josh and other researchers to lasso the full medicinal potential, if any, from Δ10-THC. And we’ll be here to bring the continuation of that story to you.
References
- Srebnik, M. et al. “Base-Catalysed Double-Bond lsomerizations of Cannabinoids: Structural and Stereochemical Aspects.” Journal of the Chemical Society, Perkin Transactions 1, issue 0, 1984, pp. 2881-2886. [journal impact factor = n/a; timed cited 3 (SemanticScholar)]
- Järbe, T. et al. “Separation of the Discriminative Stimulus Effects of Stereoisomers of A2- and A3-Tetrahydrocannabinols in Pigeons.” European Journal of Pharmacology, vol. 156, 1988, pp. 361-366. [journal impact factor = 3.170; timed cited 4 (ResearchGate)]
Image Credit: CNN-Business, Cayman Chemical