Physicochemical properties relate how a chemical interacts with certain physical environments. Understanding physicochemical properties is vital in developing effective pharmaceutically active materials. [1] Cannabis producers must analyze physicochemical parameters to ensure efficacious, standardized, and safe products. Specifically, physicochemical data indicates human/environmental toxicity, bioavailability, metabolism, and other effects. [2]
Many types of analysis are available for physicochemical properties. In 2015, Australian researchers selected 13 physicochemical tests for 664 drugs of natural origin, pointing out “causal relationships between physicochemical properties of potential drug candidates and absorption, distribution, metabolism, excretion, and toxicity (ADMET) prediction models.” [3]
Four of these tests comprise what is known as the Rule of Five (notice the multiples of 5); failing more than two of these tests indicates poor oral bioavailability:
- Molecular weight (MW): less than 500 Daltons
- H-bond donors (HBD): does not exceed 5 hydrogen bond donors
- H-bond acceptors (HBA): does not exceed 10 hydrogen bond acceptors
- Calculated logarithm of the octanol/water partition coefficient (cLogP): less than 5 [3]
The applicability of the Rule of Five is apparent for cannabis producers since bioavailability describes how much of an active substance (cannabinoids in this case) actually reach systemic circulation. Does tetrahydrocannabinol (THC) pass the Rule of Five? Let’s take a look:
- MW = 314.5 g/mol (or Da)
- HBD = 1
- HBA = 2
- cLogP = 5.648
Based on these four criteria, THC passes three. This test provides only a glimpse into the power of physicochemical data, though. The Australian researchers also tested [3]:
- Distribution coefficient (LogD7.4): measures lipophilicity at a pH of 7.4 (known as physiological pH); values higher than 3.5 are considered to have poor aqueous solubility. For reference, the logD7.4 of tetrahydrocannabinol (THC) has been recorded at 7.07. [4]
- Solubility (LogS7.4): directly measures aqueous solubility at physiological pH.
- Number of sp3 hybridized carbons/total carbon count (Fsp3): measures tetrahedral carbon atom fraction, a gauge of molecular complexity.
- Number of stereogenic carbon atoms (Chiral): measures the potential of the compound to assume asymmetric forms, or enantiomers; THC has two chiral carbon atoms and four enantiomers, although the “left-handed” (-)-trans-delta-9-tetrahydrocannabinol is the natural and common form.
- Rotatable bonds (RTB): a lower number of rotatable bonds is associated with improved oral bioavailability [7]; specifically, 10 or more reduces bioavailability. THC has four rotatable bonds.
- Ring count (RNG): number of rings in a molecule used as another metric of molecular complexity. A THC molecule contains two rings.
- Aromatic ring count (AROM): records aromatic and heteroaromatic rings; more than three aromatic rings indicates poor drug developability. [3,8] THC has one aromatic ring per molecule.
- Polar surface area (PSA): PSA measures polar atoms over total surface area. It indicates cellular permeation, including blood-brain barrier penetration; values exceeding 140 square angstroms (Å2) have low permeability. [7] THC has a PSA of 29.46 Å2. [4]
- Percent polar surface area (%PSA): reflects polar and non-polar properties. [3]
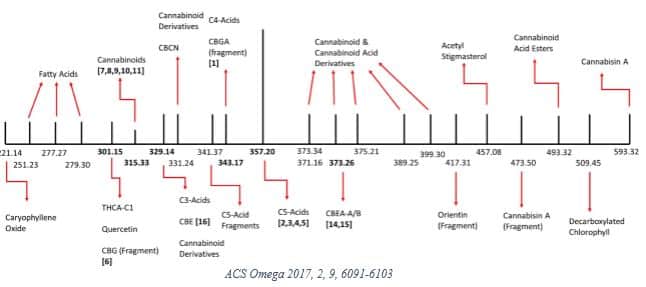
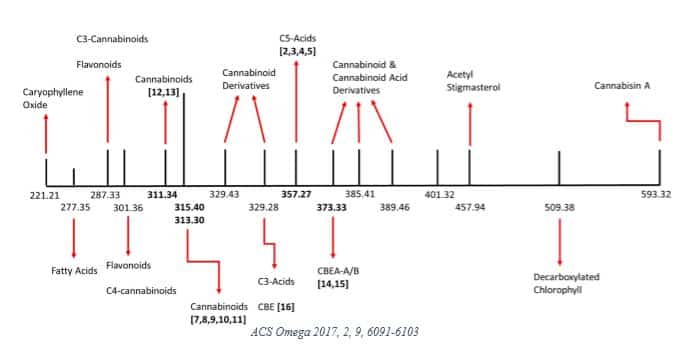
In 2017, Lewis et al [10] investigated some of the physicochemical properties in a medical cannabis extract sample from the Canadian market. They identified 22 unique MWs summarized in the graphic below.
The researchers then decarboxylated the sample and reported 16 MWs, noting that “signals corresponding to the molecular weights of 26 compounds were not found in the decarboxylated cannabis extract.”
This research reflects early attempts to characterize the complex physicochemical properties of cannabis, which undergoes significant changes depending on processing. The study authors concluded that such data is necessary to “facilitate the adoption of medical cannabis extract-based products by the wider medical community.” [10]
References
- Tietgen H, Walden M. “Physicochemical Properties.” Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays, edited by Vogel H.G., Maas J., Hock F.J., Mayer D., Springer, 2013, pp 1125-1138. Times Cited = 2 (Crossref)
- National Research Council. “Physicochemical Properties and Environmental Fate.” A Framework to Guide Selection of Chemical Alternatives, Washington (DC), National Academies Press (US), 2014.
- Camp D, et al. “Analysis of Physicochemical Properties for Drugs of Natural Origin.” Journal of Natural Products, vo.78, no.6, 2015, pp.1370-82. Journal Impact Factor = 4.257, Times Cited = 23
- Adelli GR, et al. “Effects of Specific Natural and Synthetic Cannabinoids.” Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment, edited by V.R. Preedy, Academic Press, 2017.
- Böttcher “An Additive Definition of Molecular Complexity.” J Chem Inf Model, vol.56, no.3, pp.462-470. Journal Impact Factor = 3.966, Times Cited = 21 (ResearchGate)
- Hann M, et al. “Molecular Complexity and Its Impact on the Probability of Finding Leads for Drug Discovery.” J Chem Inf Comput Sci, vol.41, no.3, 2001, pp.856-64. Journal Impact Factor = 3.966, Times Cited = 717 (ResearchGate)
- Veber DF, et al. “Molecular Properties That Influence the Oral Bioavailability of Drug Candidates.” J Med Chem, vol.45, no.12, 2002, pp.2615-23. Journal Impact Factor = 6.054, Times Cited = 2,623 (ResearchGate)
- Ritchie TJ, Macdonald SJ. “The Impact of Aromatic Ring Count on Compound Developability–Are Too Many Aromatic Rings a Liability in Drug Design?” Drug Discov Today, vol.14, no.21-22, pp.1011-20. Journal Impact Factor = 6.369, Times Cited = 329 (ResearchGate)
- Pajouhesh H, Lenz GR. “Medicinal Chemical Properties of Successful Central Nervous System Drugs.” NeuroRx, vol.2, no.4, 2005, pp.541-553. Journal Impact Factor = N/A, Times Cited = 551 (ResearchGate)
- Lewis MM, et al. “Chemical Profiling of Medical Cannabis Extracts.” ACS Omega, vol.2, no.9, 2017, pp.6091-6103. Journal Impact Factor = 2.58, Times Cited = 11 (ResearchGate)