What Solvents Must You Always Have in Stock at an Extraction Lab
Image Citation: E&E News
The cannabis extraction laboratory, as with other manufacturing plants, must maintain a regular stock of certain chemicals. Depending on the type of extraction being carried out at your facility, the most obvious chemical you’ll need at all times is your phytochemical solvent (butane/propane, CO2, ethanol). But perhaps less obvious to the laymen’s eye are the other solvents that are ubiquitously used in an extraction facility.
In a hydrocarbon or supercritical CO2 extraction facility, the next most important, and first most bureaucratically-difficult solvent to have in large quantities is ethanol. Tightly regulated by the Bureau of Alcohol, Tobacco, Firearms and Explosives, ethanol is required for the process of winterization, which precipitates and removes chlorophyll and plant waxes from the crude cannabis extract in the process of oil refinement.
Since an extraction facility is dealing with food-grade products, any time water is used, whether for mixing detergent, rinsing equipment, or cleaning filters, the water has to be purified or filtered. This is easily achieved with a reverse-osmosis water filter, which passes water across a semi-permeable membrane that filters out contaminants and reduces the hardness of the water along the way.
Rotary evaporators are still the most common way to remove ethanol from the winterized, filtered extract. The best way to run the cold finger portion of the apparatus is to immerse blocks of dry ice in isopropyl alcohol. Since carbon dioxide doesn’t have a liquid phase at atmospheric pressures, the volume of alcohol doesn’t get diluted over time. And since the melting point of isopropyl alcohol is colder than that of dry ice, it doesn’t freeze, no matter how much dry ice is shoved in there. What’s more, isopropyl alcohol is heavier than ethanol, therefore, it doesn’t evaporate quite as easily at room temperature, meaning you won’t have to replace it as often. Even so, you’ll still need isopropyl alcohol readily available for when you need to top off your cold fingers.
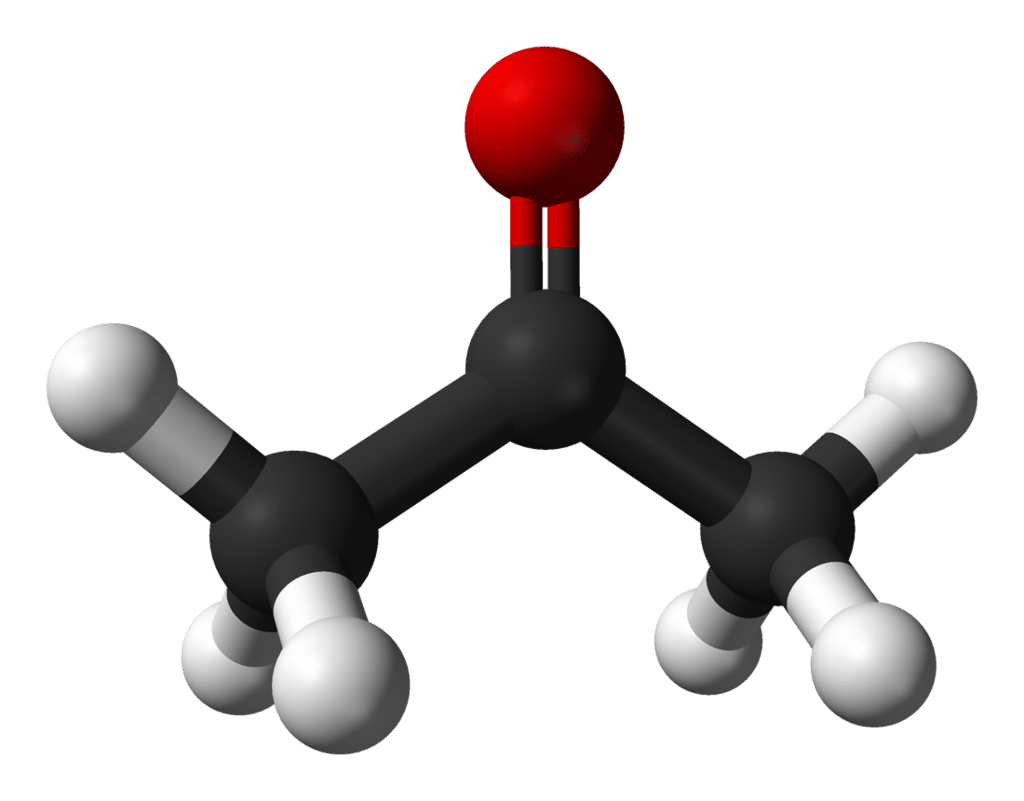
Finally, acetone is the go-to solvent for removing tough hydrophobic stains. The inside of extraction vessels, concentrate trays, decarb pots, glassware, tables; anything made of glass, ceramic, or metal can be cleaned by acetone without fear of corrosion.It is a ketone, a class of molecules defined by a carbon-oxygen double bond with two methyl groups on each side of the double bond.
Figure 1. Ball-and-stick figure of acetone, the simplest ketone. Red = oxygen, black = carbon, white = hydrogen.
Due to this chemical structure, acetone is called an aprotic polar solvent. “Polar” means it plays nicely with other polar molecules like water or ethanol. “Aprotic” means it lacks labile hydrogen atoms, so that when it solvates in liquid, it won’t shed protons and change the pH of the solution. In addition, acetone has a very low boiling point, lower than that of ethanol, and it evaporates readily in air. This makes it an ideal cleaning solvent because it quickly evaporates from the surfaces it has been used on, leaving the material liquid-free and ready for use.